Chapter 127 Revision of Aseptic Failed Total Knee Arthroplasty
Although the durability of total knee arthroplasty (TKA) with current techniques and implants is well established, failure still occurs as a result of instability, stiffness, component loosening or malposition, periprosthetic fracture, component breakage, polyethylene wear, and osteolysis. The number of primary total knee arthroplasties performed annually continues to rise rapidly.38 With a much larger population of patients having undergone primary total knee arthroplasty, the number of patients requiring revision arthroplasty will also rise, despite improvements in technique, implant design, and biomaterials.38 When failure occurs and revision is contemplated, the surgeon must recognize that revision TKA is a complex procedure that requires skill and meticulous technique to restore a predictable outcome. Preoperative evaluation should identify the cause of failure to improve the likelihood of a successful outcome.34,47 Once the cause of failure has been identified, revision surgery is performed expediently. Consideration must be given to the incision and approach, management of soft tissues, techniques of implant removal, balancing of ligaments and flexion/extension gaps, management of bone loss, tensioning and alignment of the extensor mechanism, and choice of the appropriate revision implant. The objective of revision arthroplasty is similar to that of primary surgery: to have a well-aligned limb with a stable and securely fixed implant that allows restoration of function and reduction in pain.
Indications for Revision
Mechanical Failure
Indications for revision arthroplasty include mechanical failure, malalignment, stiffness, fracture, and infection. Mechanical failure is often due to technical error at the time of primary arthroplasty.34 Mechanical failure includes aseptic loosening, polyethylene wear, osteolysis, instability, and extensor mechanism dysfunction.73 If the components are loose or have shifted position, failure is inevitable and revision surgery should be performed expediently.34 Similarly, revision is imperative with polyethylene wear-through of the tibia insert or a metal-backed patellar component. Delay will only result in additional metallic debris and massive metallic synovitis.
Osteolysis is one of the leading causes of late reoperation in patients who undergo total knee arthroplasty. The extent of osteolysis is often underappreciated with routine radiographs. Computed tomography (CT) or magnetic resonance imaging (MRI) can be obtained to more accurately image the osteolytic lesion and to determine the extent of bone loss.58 Small, asymptomatic osteolytic lesions warrant close observation and possibly medical management with bisphosphonates and calcium supplementation.27 Large, progressive, or symptomatic lesions are addressed with revision arthroplasty ranging from simple polyethylene insert exchange to full component revision with structural bone graft or porous metal augments, depending on polyethylene availability, specific implant reliability, and extent of bone loss.44
Instability is another common cause of mechanical failure of TKA. The direction of instability at the tibiofemoral articulation can occur in the coronal (varus/valgus) plane, in the sagittal (anteroposterior) plane, or as a combination of planes. Early instability may result from malalignment of the components, failure to restore the mechanical axis of the limb, or imbalance of the flexion/extension space, as is often the case with midflexion instability.49 Other common causes of early instability include intraoperative or postoperative rupture of the medial collateral ligament (MCL) or posterior cruciate ligament (PCL) with cruciate-retaining designs. Commonly, late instability occurs secondary to polyethylene wear. Asymmetrical polyethylene wear related to malalignment can result in relative lengthening of the collateral ligament on the involved side and subsequent coronal instability. In patients with cruciate-retaining knees, it is not uncommon for the PCL to elongate or attenuate. This can lead to progressive polyethylene wear and late sagittal plane instability.40 However, late sagittal plane instability is seen not only with cruciate-retaining designs. Significant wear or fracture of the tibial polyethylene post in posterior stabilized knees may result in late sagittal plane instability.55 Nonoperative management plays a small role in managing instability in TKA. Stability can often be achieved with a revision arthroplasty utilizing a posterior stabilized design. A constrained condylar design may be necessary to address collateral insufficiency or flexion and extension gap imbalance. Occasionally, a hinged design may be indicated and should be readily available.62
Extensor mechanism dysfunction remains a cause of failure in TKA. Extensor mechanism dysfunction consists of maltracking, instability, polyethylene wear, and prosthetic loosening. Unfavorable prosthetic design and error in surgical technique lead to patellar maltracking, which may result in tilt, wear, loosening, subluxation, frank instability, or patellar fracture.32 With improved prosthetic design and a better understanding of appropriate component position, the percentage of TKA failures related to extensor mechanism dysfunction is likely less than historical figures. Extensor mechanism failure responds poorly to nonoperative management and requires isolated component revision, extensor mechanism realignment, or complete component revision depending on the cause of failure.
Stiffness
Stiffness is a disabling problem following TKA. Stiffness is often associated with a decrease in functional capacity and increased pain. Before revision arthroplasty is performed for stiffness, the cause should be determined and the extrinsic sources addressed. Extrinsic sources include but are not limited to osteoarthritis of the ipsilateral hip, muscle rigidity secondary to neurologic injury, and heterotopic ossification. After exclusion of an extrinsic source, the intrinsic origin should be determined. Intrinsic causes include infection, overstuffing of the patellofemoral joint, an oversized femoral component, an excessively tight flexion or extension gap, component malposition or malrotation, a tight posterior cruciate ligament, and arthrofibrosis. If an intrinsic cause is identified and infection is ruled out, revision arthroplasty can be performed. The role of isolated arthrolysis and polyethylene component downsizing in patients with a stiff arthroplasty and well-fixed, well-aligned components remains unclear. Poor results with a high complication rate and no significant improvement in range of motion or pain were demonstrated with this approach in 7 carefully selected patients with 4-year average follow-up.2 Single-component revision may be successful in the stiff total knee with an oversized femoral prosthesis or a single malpositioned component.29,41 However, revision of both components is usually necessary.29 Full component revision is likely to provide improved results, but improvement in range of motion and level of pain has been shown to be modest in the hands of experienced surgeons despite meticulous patient selection.29,51
Periprosthetic Fracture
Periprosthetic fracture remains a problematic complication following arthroplasty. It is estimated that 0.3% to 2.5% of patients will sustain a periprosthetic fracture as a complication of TKA.17 Patient-specific factors, including rheumatoid arthritis, osteopenic bone, and osteolysis, and technique-specific factors, such as anterior femoral cortical notching, have been implicated as potential causes of periprosthetic fracture. Frequently, fractures occur in the supracondylar area above a well-fixed implant.33 Fractures of the tibia are much less common and frequently are associated with implant loosening.20 In general, patients with fractures around loose implants are best treated with revision TKA, whereas those with fractures around well-fixed implants should be considered for open reduction and internal fixation.17
Infection
Deep infection remains one of the most devastating and challenging complications of TKA. It is estimated that by 2030, 65.5% of all revisions will be performed secondary to infection.38 Currently, the risk of postoperative infection after TKR is 0.4% to 2.0%.31 Appropriate treatment for acute infection remains debatable and depends on organism virulence, host factors, and time from onset to surgical intervention.61,66 Chronic infection is treated most appropriately with two-stage reimplantation, including removal of components, débridement, and placement of a cement spacer, followed by 6 to 8 weeks of intravenous antibiotics and reimplantation once the infection has been eradicated31 (Fig. 127-1). Complete discussion of the management of periprosthetic infection is beyond the scope of this chapter.
Preoperative Assessment
History and Physical Examination
The wound and skin should be carefully evaluated for evidence of local infection or peripheral vascular disease. Meticulous palpation for point tenderness can lead to a diagnosis of tendinitis, bursitis, or cutaneous neuroma.12 Physical examination of the hip and spine can reveal sources of referred pain, including ipsilateral hip arthritis or radiculopathy. Evaluation of the knee with regard to range of motion, stability, alignment, patellar tracking, and the presence of an effusion may provide additional evidence of local infection, malalignment, instability, stiffness, or extensor mechanism dysfunction.
Laboratory Evaluation
All patients presenting with a painful TKA should have a complete blood count with differential (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). The CBC is often normal even in the presence of infection. Any elevation in laboratory values should raise the clinician’s suspicion for infection. Routine use of additional laboratory tests, including serum interleukin-6, procalcitonin, and tumor necrosis factor-alpha, in making the diagnosis of infection has yet to be implemented, but may play a role in the near future.6
Aspiration is advisable whenever joint fluid is present.12 The aspirate should be examined for signs of purulence, bleeding, metallic or polyethylene debris, or change in viscosity. The fluid is then sent for cell count, gram stain, and aerobic, anaerobic, and fungal culture. A synovial fluid leukocyte differential of greater than 65% neutrophils has a sensitivity of 97% and a specificity of 98% for diagnosing prosthetic joint infection; a leukocyte count greater than 1.7 × 103/µL has a sensitivity of 94% and a specificity of 88%.67 Ghanem and associates23 reported similar results and recommend a cut-off of greater than 64% neutrophils and a leukocyte count greater than 1.1 × 103/µL, which demonstrated a combined positive predictive value of 98.6%.23
Radiographic Evaluation
Routine standing anteroposterior (AP) and lateral radiographs should be obtained and evaluated for component position, fixation, and sizing. Any evidence of osteolysis, polyethylene wear, component failure, loosening, or migration is noted. The most recent radiographs are compared with initial postoperative films to address concerns of subtle component migration, progressive radiolucent lines, or progressive osteolysis. A Merchant view is also essential in evaluating the patellofemoral articulation and extensor mechanism tracking. Obtaining a full-length hip-to-ankle film has several advantages. This film can be evaluated for sources of referred pain from the ipsilateral hip, including degenerative joint disease and stress fracture. It allows more accurate assessment of alignment of the involved limb and can detect distant osseous problems such as malunion, tumor, stress fracture, orthopedic hardware, or an adjacent joint arthroplasty that may be the source of pain or may interfere with a planned revision.12 Additional radiographic views may be warranted in specific scenarios. Fluoroscopically positioned radiographs can be used to image the implant fixation interface tangentially and allow the diagnosis of subtle component loosening.18 Oblique radiographs have been shown to enhance visualization of the periprosthetic bone and to facilitate diagnosis of early osteolysis, especially with posterior stabilized implants.48 Stress radiographs are not routinely needed, but may assist in diagnosing subtle ligamentous instability or PCL deficiency.14
Advanced Imaging
Computed tomography (CT) is used to more accurately diagnose and size periprosthetic osteolysis related to polyethylene wear. This can facilitate preoperative planning with regard to the management of bone loss during revision arthroplasty. Reish and colleagues58 demonstrated that standard radiographs detected 17% of osteolytic lesions diagnosed by multidetector CT in 31 patients. CT is an effective and accurate way to measure tibial and femoral component rotation.58 This may be most appropriate in the preoperative evaluation of patients with extensor mechanism complications, including patellofemoral pain, excessive tilt, maltracking, subluxation, or dislocation.
Recent modifications of MRI pulse sequence parameters have permitted imaging of arthroplasty with significantly less artifact. MRI allows imaging of the surrounding soft tissue envelope, including nerves, tendons, and ligaments. As well, MRI can be a useful tool to detect and quantify particle disease, osteolysis, synovitis, and prosthetic infection.54 The exact role of MRI in evaluating the painful TKA has not yet been determined; however, it is likely that MRI will play a larger role in the future as imaging abilities continue to improve.
Nuclear medicine scans play an undefined role in evaluating the painful or failed TKA. Commonly used scans include the Tc-99m–labeled bone scan, the 111gallium Ga-67 scan, the indium-labeled white blood cell scan, and the sulfur colloid bone marrow scan. Positive scans indicate loosening, stress fracture, infection, or complex regional pain syndrome. Nuclear scans typically provide high sensitivity but variable specificity. A Tc-99m–labeled bone scan may be positive up to 2 years following a successful arthroplasty that may limit its role in evaluation of the recently postoperative patient. Smith and associates64 reviewed the use of 99Tcm-MDP in evaluating 80 painful TKAs. They demonstrated low specificity (75.9%) and a positive predictive value (64.9%). Specifically, 33% of patients with an abnormal scan had a normal TKA with further follow-up. However, a negative bone scintigram has proved reassuring. In Smith’s study, the sensitivity and negative predictive value were 92.3% and 95.0%, respectively. 111Indium-labeled white blood cell scans are used most often in evaluation for infection. Rand and colleagues57 evaluated 18 infected and 20 noninfected TKAs with 111indium scan. They demonstrated a sensitivity and specificity of 83% and 85%, respectively, along with a diagnostic accuracy of 84%. Similarly, Scher and coworkers60 observed 84% accuracy and a 95% negative predictive value for the prediction of infection in 143 arthroplasty patients evaluated with 111indium leukocyte scan. As demonstrated, a positive 111indium scan is by itself nonspecific and can be positive because of marrow redistribution around a prosthesis. Therefore, the 111indium scan has been combined with a Tc-99m sulfur colloid scan to scan bone marrow. Incongruent uptake of the two is highly suggestive of infection.64 Palestro and associates52 demonstrated greater diagnostic accuracy (95%) with combined labeled leukocyte and sulfur colloid marrow imaging compared with that of labeled leukocyte scintigraphy alone (78%).
Preoperative Planning
Preoperative planning is essential for successful revision surgery. The exact mode for failure of the prior arthroplasty must be identified.34,73 The diagnosis of infection in the vast majority of cases is established prior to the procedure, so intraoperative “surprises” are rare.73 The original operative report is obtained and reviewed whenever possible. This provides information regarding the previous approach, prior soft tissue management, including releases, and implant-specific information, including manufacturer, design, and size. This is particularly important if single-component revision is being entertained. Thought is given to the type of prosthesis and the amount of constraint that will be required for revision.62 Any special components must be ordered in advance. The surgeon attempts to quantify the extent of bone loss and osteolysis present with the knowledge that it is often underestimated. The need for structural bone graft, augments, wedges, porous metal metaphyseal cones, and stems is anticipated, and they are made readily available during the revision. Preoperative templating for selected revision components can be helpful. This is essential in cases where extra-articular deformity or osseous pathology is present that may require osteotomy or may interfere with stem fixation. Revision components should be modular to allow intraoperative attachment of augments, wedges, and stems. The revision proceeds more predictably when the cruciate ligaments are excised and both posterior stabilized and constrained condylar designs are available. Ligament stability and integrity of the extensor mechanism are assessed. If ligament stability is compromised, at the time of revision a hinged prosthesis is kept readily available. A compromised soft tissue envelope due to impaired skin viability or previous incision may warrant a plastic surgery consultation; occasionally, soft tissue expansion or a soft tissue flap will be required.25,45
Surgical Technique
Exposure
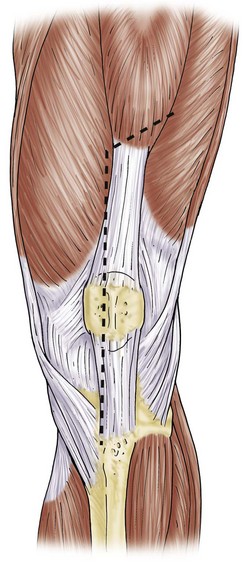
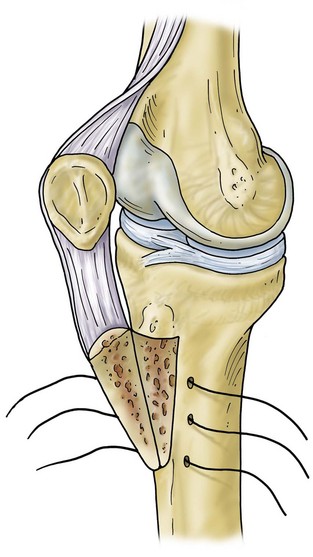
The surgical approach most often utilizes the previous surgical incision. In cases with multiple longitudinal prior incisions, the most lateral and anterior incision is used to preserve the blood supply to the medial aspect of the lateral skin flap.36 Attempts to maintain a minimum skin bridge of 6 cm between parallel incisions are recommended. Previous transverse incisions that cannot be avoided are crossed at 90 degrees if possible, but certainly at no less than 60 degrees. Soft tissue expanders can be considered in cases with multiple crossing incisions or densely adherent soft tissue.45 Subcutaneous dissection is carried out in a limited manner, and flaps are kept as thick as possible to avoid ischemia. A medial parapatellar arthrotomy is performed. Synovial fluid is obtained as the first intraoperative culture. In revision arthroplasty with good preoperative motion, a medial subperiosteal exposure that allows the tibia to be externally rotated and anteriorly subluxed is usually sufficient for exposure. This is incorporated into a medial release if needed for soft tissue release and balancing. In revision for infection or arthrofibrosis, or during two stage reimplantation, a quadriceps snip may be anticipated and is performed early to prevent injury to the tibial tubercle and extensor mechanism63 (Fig. 127-2). The quadriceps snip is a versatile exposure that is used in a majority of revisions requiring extensile exposure; it does not require alteration of the postoperative weight-bearing protocol. When quadriceps snip does not allow adequate exposure, tibial tubercle osteotomy or a “banana peel” release of the patellar tendon can be considered39,71 (Fig. 127-3). A long osteotomy as described by Whiteside and Ohl71 is particularly useful in patients with marked patella baja, or to assist with removal of long cemented stems and well-fixed ingrowth components. A “step-cut” is performed at the most proximal aspect of the osteotomy. This allows more secure fixation and helps prevent proximal escape of the fragment. Care is taken to maintain the lateral soft tissue attachments to the osteotomized bone and to hinge the osteotomy open. This facilitates closure and maintains fragment vascularity. The fragment is repaired utilizing cerclage wires or two screws.71 With secure fixation, the postoperative weight-bearing protocol does not have to be altered. An alternative technique for additional exposure is V-Y quadricepsplasty.1,63 V-Y quadricepsplasty provides excellent exposure and allows lengthening of the extensor mechanism if needed. Quadicepsplasty necessitates postoperative immobilization in extension and may result in extensor lag. For patients with rigid deformity and arthrofibrosis, a femoral peel may be necessary. Because this procedure involves complete release of the medial and lateral supporting structures, use of a constrained design will be necessary.
Fixed angular deformities are often encountered during revision arthroplasty and are addressed during the exposure. A fixed varus deformity is corrected with subperiosteal release of deep and superficial portions of the MCL and the pes anserine insertion.37 The distal insertion of the superficial MCL is elevated subperiosteally in an incremental fashion with a straight 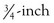 osteotome. Finally, while the tibia is externally rotated, the semimembranosus and posterior capsule are released off the posteromedial aspect of the tibia.37 This results in skeletonization of the proximal medial tibia. A fixed valgus deformity is less common in the revision setting, but if encountered, it must be addressed. Mild valgus deformity of less than 20 degrees may be addressed with the lateral “pie-crust” technique.10 Severe valgus deformities of greater than 20 degrees necessitate complete release of the lateral supporting structures from the femoral condyle with a subperiosteal peel or a lateral epicondylar osteotomy.
osteotome. Finally, while the tibia is externally rotated, the semimembranosus and posterior capsule are released off the posteromedial aspect of the tibia.37 This results in skeletonization of the proximal medial tibia. A fixed valgus deformity is less common in the revision setting, but if encountered, it must be addressed. Mild valgus deformity of less than 20 degrees may be addressed with the lateral “pie-crust” technique.10 Severe valgus deformities of greater than 20 degrees necessitate complete release of the lateral supporting structures from the femoral condyle with a subperiosteal peel or a lateral epicondylar osteotomy.
Removal of Components
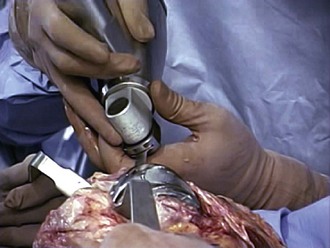
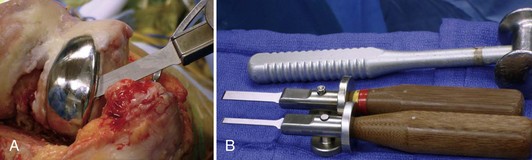
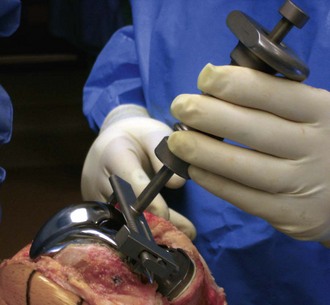
Revision operations are being performed increasingly for reasons other than loosening. Removal of well-fixed components can be difficult, especially if they are porous coated or have long cemented stems. Initially, all soft tissue is cleared from the bone/cement/prosthesis interface. Special instruments can facilitate component removal. If a modular tibial component is present, the polyethylene insert is removed first to open both flexion and extension spaces. This aids in obtaining additional exposure. The polyethylene insert is removed using manufacturer-specific tools for extraction, or by passing a straight osteotome between the insert and the tibial component. The femoral component is subsequently removed. A microsagittal saw blade is placed along the cement/prosthesis interface and is moved parallel to the component to avoid cutting into the bone (Fig. 127-4). Thin flexible osteotomes are then passed around the periphery of the component to separate the component at the cement/prosthesis interface and leave the underlying bone intact (Fig. 127-5A and B). Once the adhesion between component and cement is broken, a femoral component extraction tool is used to gently remove the femoral prosthesis (Fig. 127-6). If the procedure is performed properly, the cement is left behind still attached to the bone with minimal bone loss from implant removal. The cement is then removed by cracking it with a small osteotome in a mosaic pattern.
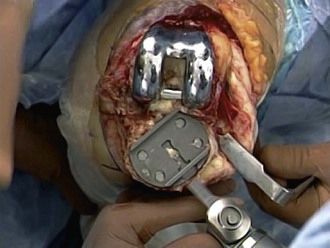
The tibial component is addressed next. All polyethylene components are separated from the tibial surface with a microsagittal saw that cuts across any polyethylene pegs or stems that are subsequently removed. Metal-backed tibial components are approached in a similar manner as the femoral component. The microsagittal saw is passed beneath and parallel to the tibial component (Fig. 127-7). Caution should be used to avoid digging into the tibial bone and causing unnecessary iatrogenic bone loss. Due to exposure, separating the tibial component from the cement mantle is most difficult on the posterolateral aspect of the tibia. Tibial exposure is improved by external rotation of the tibia and release of the semimembranosus and posterior capsule. The posterolateral aspect of the component is then reached by passing the microsagittal saw and thin flexible osteotomes under the posteromedial aspect of the tray to the posterolateral side. Once the bond is broken between the cement and the component, a manufacturer specific extractor or the femoral component extractor is used to gently lift the component from the tibia. An osteotome is used to crack the remaining cement in a mosaic pattern.
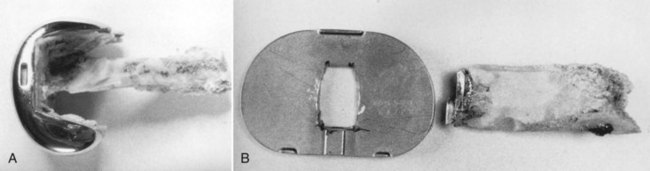
A well-fixed, compatible patellar component that tracks well is left in place.3,28 All polyethylene patellar components are removed by cutting between the cement/component interface with an oscillating saw. The saw cuts across the pegs, which are subsequently removed with a small burr or drill bit. Metal-backed patellar components are more difficult to remove. A high-speed diamond-edged saw may be necessary to remove a well-fixed uncemented metal-backed patellar component13 (Fig. 127-8).
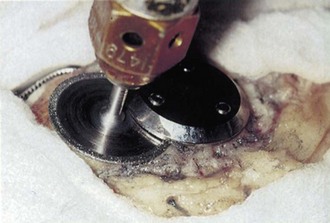
Figure 127-8 Removal of a well-fixed metal-backed patellar component with a diamond-edged saw blade.
After the components are removed, the bone surfaces are cleaned of cement, debris, and granulation tissue (Fig. 127-9). The bone ends are “freshened-up” in preparation for cement fixation of the revision component. In the revision setting in which infection has been ruled out, well-fixed cement in the canals that does not impact component stem placement can be left in place to avoid unnecessary iatrogenic bone loss or perforation of the canal. Removal of a cemented porous-coated prosthesis can be a difficult task, especially with stems designed for bone ingrowth. It may be necessary in this scenario to disassemble the components to gain access to the stems (Fig. 127-10).
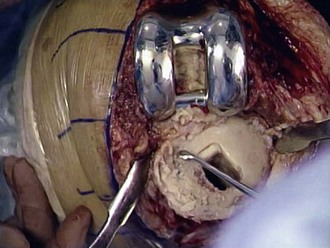
Figure 127-9 After removal of the components, retained cement and debris are removed with curettes, osteotomes, and a rongeur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree