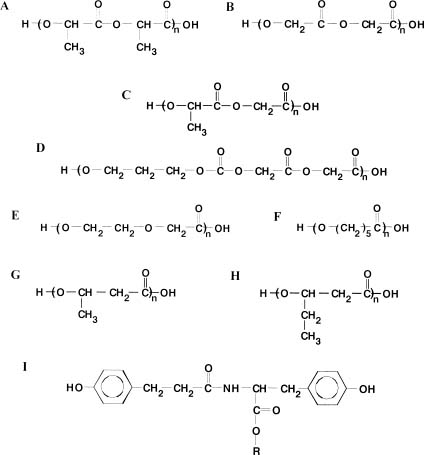
Chapter 23 The treatment of bone fractures in patients with osteoporosis is a problem. Conservative techniques involving casts or skeletal traction are associated with well-appreciated drawbacks. Standard metal internal fixation devices fail frequently when applied in brittle, osteoporotic cortical bones. Hence, methylmethacrylate (MMA) cement is often used to improve the purchasing power of screws and the stability of fixation of osteoporotic bone fractures.1–20 The main disadvantages of this treatment modality are associated with the high temperature developed when MMA monomer sets, bone necrosis, release of toxic residual monomer, and the difficulty of removal of solid polymethylmethacrylate (PMMA) in the case of infection. An alternative to MMA cement for the reinforcement of osteoporotic bones could be provided by ceramic cements based on calcium phosphates. These cements solidify without the exothermal effect, can be osteoconductive, and can be produced with mechanical properties comparable to those of MMA cement.21–30 Yet another approach to augment screw purchase in osteoporotic bone might be to use medullary inserts produced from resorbable polymers.31,32 Biomedical polymers for implants should not have adverse effects upon the recipient of the device, should not induce adverse inflammatory or foreign body reactions, and should not be carcinogenic, mutagenic, teratogenic, or toxic. Biomedical polymers should be pure and reproducibly produced, and have final properties adequate for the intended application. Hence, flexural strength, bending strength, shear strength, Young’s modulus, fatigue resistance, and wear resistance are critical for internal fixation implants. Tensile strength and tear, burst, and fatigue resistance are essential for implants intended for use in the reconstructive surgery of soft tissues and in cardiovascular surgery. The physical and chemical properties of implantable materials should not undergo changes in the biological environment, unless they are designed as resorbable or degradable materials.33 The potential of using resorbable polymers for medical devices has been recognized since the early 1970s. At present, the main clinical applications of resorbable polymers are for wound closure, internal fixation of bone fractures, tissue engineering, and drug delivery devices, to mention just a few. There are various definitions of resorbable polymers. In general, they are polymers deliberately designed to be degraded in vivo to nonharmful by-products. Such by-products are usually present in the body as metabolites and/or tissue components. The former are finally eliminated from the body by normal metabolic routes; the latter can be incorporated into the tissues. In vivo degradation can be described as the structural and chemical changes in the implant resulting from the interaction between components of the living tissue and the implant material, which acts as a foreign body at the implantation site. Resorbable polymers can be of natural or synthetic origin. The latter, mainly commercial polyhydroxyacids, are more suitable for implants subjected to the action of mechanical stress or load.34–59 Resorbable polymers for implants should be commercially available in high-purity, reproducible grades. The batch variations may have an impact on material processing, final implant performance, and biocompatibility. Polymer processing and implant sterilization should not affect its molecular or mechanical properties. The polymers chosen for implants should have proven biocompatibility in soft tissue and/or in bone. The time for complete material resorption in vivo should be known to ensure that the particular device intended for the given application is produced from the material with an optimal or at least acceptable resorption rate. This means that the rate of implant degradation should not be too slow to avoid prolonged inflammation, yet it should not be too fast to allow for an effective metabolism of the degradation products formed upon implant degradation. Ideally, the resorbable implant should maintain its mechanical properties and remain in place only for the time required for tissue healing.34 Commercial resorbable polymers are primarily polyhydroxyacids, including various polylactides based on L-lactide, D-lactide, meso-D,L-lactide, racemic LD-lactide; polyglycolide, copolymers of lactides with glycolide, ε-caprolactone, or trimethylene carbonate; copolymers of glycolide with trimethylene carbonate or ε-caprolactone, poly (p-dioxanone), and to a lesser extent poly (hydroxybutyrate) and poly (hydroxybutyrate-co-hydroxyvalerate) with various content of the valerate unit (Figs. 23–1A through I).34,38–41 In addition, poly (ortho esters),43 polyanhydrides, polyesteramides, and poly (tyrosine carbonates)44 are considered as materials for implants. More recently, triblock copolymers of L-lactide, D-lactide, and glycolide have been pursued as materials for implants for cranio- and maxillofacial applications. FIGURE 23–1 Chemical structures of selected resorbable polymers. (A) Polylactide; (B) polyglycolide; (C) poly (lactide-co-glycolide); (D) poly (glycolide-co-trimethylene carbonate); (E) poly (dioxanone); (F) poly (ε-caprolactone); (G) poly (hydroxybutyrate); (H) poly (hydroxyvalerate); (I) tyrosine-derived diphenolic diol used in the syntheses of polycarbonates. Polymeric materials placed in liquids undergo changes due to chemical and electrical interactions. These changes can be reversible if only secondary bonds are affected, or irreversible when upon hydrolysis the covalent bonds are cleaved. In the latter case, the products of degradation are small-chain fragments and monomers. Degradation of polyhydroxyacids in the aqueous media proceeds via a random, bulk hydrolysis of ester bonds in the polymer chain with the formation of carboxylic acids. Degradation in vivo may be enhanced by the presence of tissue enzymes, peroxides, cellular activity, lipids, and traces of elements. Lysis may also contribute to the degradation process. Carboxylic acids are finally transformed into carbon dioxide and water (Krebs cycle). The chain scission takes place in the amorphous regions first as they are more permeable to liquids, followed by degradation of the crystalline regions. This is accompanied by a significant reduction in mechanical properties. For semicrystalline polymers the crystallinity of the material initially increases, to be reduced again at the final stage of degradation. If polymers contain impurities, these are released into the surrounding tissue together with the degradation products. Impurities can be accumulated at the implantation site, transported by macrophages, or moved passively through the tissue and the circulatory system. The amount and biological quality of degradation products will finally affect the tissue reaction to the implant.35–40,42,45–59 Degradation of polymeric implants in vitro and in vivo depends on a number of factors related to their inherent characteristics and/or properties achieved upon processing. Chemical structure, chain regularity, molecular weight, polydispersity, surface chemistry, hydrophilicity, and glass transition temperature belong to the first category. The molecular orientation, overall crystallinity, presence of pores, defects, size, shape, presence of impurities, and additives are introduced upon processing. Yet more factors affecting degradation include load and stress acting on the implants, the aggressiveness (pH) and temperature of the environment, the site of implantation (vascularity), and bacterial contamination. In general, the rate of degradation of resorbable polymers decreases with increasing chain regularity, molecular weight, chain orientation, crystallinity, and number of cross-links. The presence of monomer and catalyst residues, voids, and hydrophilic moieties facilitates polymer degradation. The rate of degradation is also mass-dependent (i.e., the greater the amount of polymer, the slower its clearance).34 Although the body of published literature on in vitro and in vivo degradation of resorbable polymers is rich, comparison of the data is difficult. Test samples from polymers having the same chemical composition are often claimed to lose mechanical properties at different rates and resorb completely at different times.35–40,42,45–59 These discrepancies may result from the fact that samples used by various investigators differed in molecular weights, overall crystallinity, chain orientation, the presence of voids, and purity. Implants from thermoplastic resorbable polymers for trauma and orthopaedic applications are mainly produced using common melt-processing methods such as injection-molding, extrusion, and compression-molding.
RESORBABLE IMPLANTS AS A MEANS
OF AUGMENTING METAL PLATE
FIXATION IN OSTEOPOROTIC BONE
GENERAL REQUIREMENTS FOR BIOMEDICAL POLYMERS
RESORBABLE POLYMERS FOR IMPLANTS
CRITERIA FOR SELECTING RESORBABLE POLYMERS
COMMERCIAL RESORBABLE POLYMERS
DEGRADATION OF RESORBABLE POLYMERS
PROCESSING OF RESORBABLE POLYMERS INTO IMPLANTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree