Release of the First Dorsal Compartment
Marc E. Umlas
Richard H. Gelberman
Indications
Surgical release of the first dorsal compartment is indicated following failure of conservative treatment of de Quervain disease. Initially, patients are treated by a trial of splinting and corticosteroid injection of the first dorsal compartment. Repeat trials of nonoperative treatment, including up to three injections, are occasionally indicated. If the patient fails to improve, operative release is elected.
Contraindications
No direct contraindications to surgery exist, but it is important to explore fully other possible local conditions (e.g., basal joint arthritis, triscaphoid arthritis) as explanations for failure of conservative treatment.
Preoperative Planning
Initial patient evaluation includes history, physical examination, and plain radiographs of the wrist and hand. Patients present with complaints of pain and swelling over the dorsal radial aspect of the wrist. Wrist pain exacerbated by thumb motion is classic, but inconsistently noted by the patient. A thorough occupational history is necessary, because chronic overuse has been implicated in the disease process. It is important to question the patient for associated conditions. Arons (1) in 1987 noted a high incidence of “generalized nonarticular rheumatism of the upper extremity” in patients presenting with de Quervain disease. This included fasciitis, tendonitis, bursitis, and chronic ligament and muscle strain. A thorough medical history is important as well, with attention to concurrent rheumatologic diseases and diabetes.
Begin physical examination with an evaluation of both hands. Tenderness over the contents of the first dorsal compartment is elicited. Perform Finkelstein’s test, which is fairly specific for de Quervain disease. To perform this test, have the patient place the thumb into the ipsilateral palm and grip the thumb tightly. Then place the patient’s wrist into a position of forced ulnar deviation. Pain directly over the first dorsal compartment signifies a positive test result. It is important to differentiate de Quervain disease from both basal joint arthritis and triscaphoid arthritis. Perform a basal joint grind test, which is positive in basal joint arthritis and normally negative in de Quervain disease. Differentiate triscaphoid arthritis by direct palpation. Perform a neurologic examination to rule out concomitant carpal tunnel syndrome.
All patients have plain radiographs taken of the wrist and hand. Attention is drawn to bony abnormalities in the region of the first dorsal compartment. Additionally, note basal joint arthritis and triscaphoid degenerative changes.
Once the diagnosis has been made, treat the patient nonoperatively. Initiate a trial of injection and splinting. Prepare the dorsal radial aspect of the wrist with Betadine. Inject a mixture containing
40 mg of methylprednisolone acetate (Depo-Medrol; Upjohn, Kalamazoo, MI) and 1% solution of lidocaine without epinephrine into the tendon sheath of the first dorsal compartment through a 22-gauge needle. Introduce the needle 1 cm proximal to the tip of the radial styloid process and angled it distally at 45 degrees to the longitudinal axis of the lateral aspect of the forearm (Fig. 43-1). Note filling of the tendon sheath distal to the annular ligament of the first dorsal compartment. Fit the wrist with a polyethylene thumb spica splint with the wrist in 20 degrees of dorsiflexion and the metacarpophalangeal and interphalangeal joints of thumb in extension. The patient, maintained in the splint for 3 weeks, is allowed to remove it for bathing. After 3 weeks of immobilization, unrestricted activity is allowed. A total of 6 weeks following injection must elapse before the success of the injection is judged. Failure of nonoperative treatment is indicated by persistence of first dorsal compartment tenderness, a positive Finkelstein’s test, and continued restricted use of the hand because of persistent symptoms. Having failed a trial of conservative treatment, consisting of one to three injections, the patient is considered for operative release of the first dorsal compartment.
40 mg of methylprednisolone acetate (Depo-Medrol; Upjohn, Kalamazoo, MI) and 1% solution of lidocaine without epinephrine into the tendon sheath of the first dorsal compartment through a 22-gauge needle. Introduce the needle 1 cm proximal to the tip of the radial styloid process and angled it distally at 45 degrees to the longitudinal axis of the lateral aspect of the forearm (Fig. 43-1). Note filling of the tendon sheath distal to the annular ligament of the first dorsal compartment. Fit the wrist with a polyethylene thumb spica splint with the wrist in 20 degrees of dorsiflexion and the metacarpophalangeal and interphalangeal joints of thumb in extension. The patient, maintained in the splint for 3 weeks, is allowed to remove it for bathing. After 3 weeks of immobilization, unrestricted activity is allowed. A total of 6 weeks following injection must elapse before the success of the injection is judged. Failure of nonoperative treatment is indicated by persistence of first dorsal compartment tenderness, a positive Finkelstein’s test, and continued restricted use of the hand because of persistent symptoms. Having failed a trial of conservative treatment, consisting of one to three injections, the patient is considered for operative release of the first dorsal compartment.
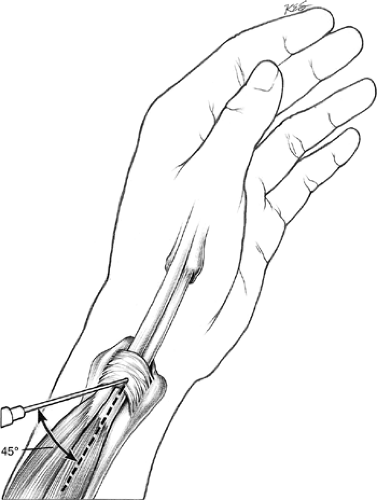 Figure 43-1 Inject local anesthetic and steroid solution into the first dorsal compartment of the wrist. |
Witt et al. (2) reported 38% unsatisfactory results from steroid injection and splinting. No significant association between the outcome of nonoperative treatment and sex, hand dominance, age, or duration of symptoms was noted.
Patients referred for operative release are scheduled in the same day at the surgical unit of for release of the first dorsal wrist compartment under local anesthesia. They are informed about the nature of the procedure and possible complications, including numbness in the distribution of the radial sensory nerve, painful neuroma formation in the distribution of the radial sensory nerve, and failure of the operation to relieve symptoms of pain and tenderness.
Preoperative Preparation
Position the patient supine with the involved upper extremity placed on a hand table.
Place an upper arm inflatable tourniquet cuff. Use standard skin preparation and draping.
Apply an Esmarch bandage to the arm; make four wraps of the hand with the Esmarch and then progress proximally to cuff (Fig. 43-2).
Inflate the tourniquet to 250 mm Hg and remove the Esmarch bandage.
Carefully palpate the landmarks (Fig. 43-3).
Technique
Mark the radial styloid and skin incision with methylene blue skin marker (Fig. 43-4).
Draw a 2-cm transverse skin incision 1 cm proximal to the radial styloid process, directly over the first dorsal compartment.
Infiltrate the skin and subcutaneous tissues with 1% lidocaine without epinephrine (Fig. 43-5).
Incise the skin with a no. 15 scalpel along the predrawn line (Fig. 43-6).
Carry out subcutaneous dissection with blunt-tipped scissors down to the level of the extensor retinaculum (Fig. 43-7).
Identify and protect branches of the radial sensory nerve (Fig. 43-8).
Incise the sheath overlying the extensor pollicis brevis (EPB) and abductor pollicis longus (APL) in a longitudinal fashion at its dorsal margin (Fig. 43-9). This leaves a palmarly based flap of retinaculum to prevent palmar subluxation of the tendons with wrist flexion (3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree