Rehabilitation Robotics
H. I. Krebs
B. T. Volpe
S. Hesse
A. C. Lo
J. Stein
N. Hogan
INTRODUCTION
Rehabilitation robotics may be organized under four major mechatronic clusters, namely, (a) robotic-assistants, (b) prosthetics, (c) orthotics, and (d) therapeutic robotics. Robotic-assistants comprise devices that perform a task in lieu of the person. Prosthetics comprises devices that substitute for limb function. Orthotics aims at augmenting weak or paralyzed limbs by supporting loads and assisting or resisting relative motion between body segments. Finally, therapeutic robotics encompasses an emerging class of interactive robots that support and enhance the clinician’s task of facilitating recovery, delivering therapy, and evaluating patient progress. There are other ways to organize and characterize the scope of rehabilitation robotics. For example, another traditional way separates the devices into two broader categories: the intent to assist the patient in coping with the environment (assistive technology) and the intent to assist the clinician in delivering therapy and facilitating recovery (therapeutic technology). The first three mechatronics clusters mentioned earlier, robotic-assistants, prosthetics, and orthotics, fall under the assistive technology scope while the fourth, therapeutic robotics, falls under its namesake category. More recently, some researchers have organized rehabilitation robotics under a mixed classification system which is aggregated according to user group characteristic, to the source of inspiration for the mechatronics design, and finally according to the goal of the mechatronics system. For instance, many recent conferences are organized under three tracks, namely gerontechnology (user group characteristic), biorobotics (source of design inspiration), and neurorobotics (harness neurorecovery or neuron connectivity). It must be noted that part of the rationale for the recent spurt of neologism around rehabilitation robotics has to do not only with a sense of excitement and perceived opportunities of growth but also with the strong desire to distance itself from old labels and clearly delineate a new crossdisciplinary, biomedical and engineering endeavor.
THE PROBLEM
There is reason for this optimistic view for the rehabilitation robotics field. The demand for rehabilitation services is significant and is expected to grow apace with the graying of the population. For instance, consider cerebral vascular accidents (CVA) or stroke: according to the World Health Organization (WHO), every year 15M persons have a stroke, with a third of them left permanently disabled (1). We estimate that the number of persons who have survived a stroke at any given time is nearly ten times the yearly survival incidence. While there is no reliable data, one can estimate an annual need for rehabilitation services on the order of 50M patients worldwide. Of course, these figures are just “back-of-the-envelope” estimates and must be checked against accurate statistics and local variability. Nevertheless, these coarse estimates highlight the sizeable need and opportunity to deploy technologies such as robotics not only to promote recovery but also to assist a person to cope with residual impairment and disability. Robotics can revolutionize rehabilitation medicine by harnessing technology to assist, enhance, and quantify recovery and to ameliorate the quality of life and independence of the elderly and disabled. In this chapter, we focus exclusively on therapeutic robotics (for more information on assistive technology, orthotics, and prosthetics see (2,3)).
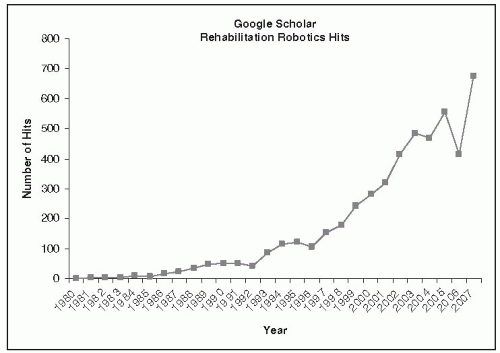
Therapeutic robots are a new class of clinical tools for evaluating patients and delivering meaningful therapy that enables an overdue transformation of rehabilitation clinics from preindustrial manual operations to technology-rich activities. Applications of robots to therapy are fairly recent, with the premier engineering journal in the field, the IEEE Transactions on Rehabilitation Engineering, coming to life only in 1992 (renamed in 2001 to IEEE Transactions on Neural Systems and Rehabilitation Engineering). In the last 10 years, the field of rehabilitation robotics has undergone vigorous growth. Borrowing an appropriate neologism, we “googled” Google Scholar with the words “rehabilitation robotics” and tracked yearly growth of academic activity in the field (Fig. 83-1). These data should be viewed with appropriate caution, particularly during the early years. But recent robust growth shows no sign of slackening and reflects a real phenomenon that is far from abating.
To keep pace with this fast-growing field is a daunting task, and our goal in this chapter is not to present a survey of the different existing devices, which would be doomed to obsolescence because of the pace of change. That task would better be achieved by tracking publications in the top journals in the field and the meta-analysis of results (4, 5, 6, 7, 8, 9). Here, we attempt to highlight some basic robotic differences, present some limited outcomes employing both upper and lower extremity robotic devices, discuss some common concerns with the technology, and finally discuss potential mechanistic views of why it might actually work.
DIFFERENT ROBOTS: WHAT ARE THE DIFFERENCES?
This increase in activities has resulted in a multitude of different designs, different devices, and different choices for clinicians. Rather than make an exhaustive comparison of different designs, our goal herein is to discuss the critical differences among therapy robots. However, it is very important to stress that ultimately only exhaustive clinical results can actually determine what works, what does not, what works better, when to deliver therapy during the course of the illness or recovery, and, most importantly, for whom.
End-Effector Versus Exoskeletal Designs
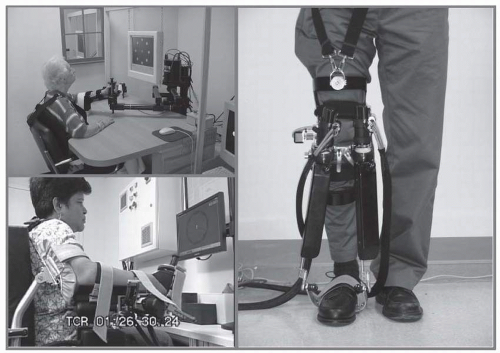
The whole gamut of devices can be parsed into two broad categories of “end-effector” or “exoskeletal” designs (Fig. 83-2). Care must be exerted to understand the design trade-offs. Exoskeletal devices allow us to estimate with reasonable accuracy the kinematics of the human joints, but this advantage comes at a cost as it takes considerable time to properly adjust the lengths of the device links to each patient’s limb lengths. Note that proper collocation of the device and human joints might be necessary in some designs to prevent injury. Another advantage of an exoskeletal design is that it allows for a smaller package when therapy requires large human joint angle displacement. End-effector designs for very large human joint angle displacement require larger devices. Conversely, because the actuators (motors) are typically located at the joints of an exoskeletal device which must carry the weight of each actuator, large transmission reduction ratios are typically employed to reduce the weight of these motors, which lead to intrinsically large impedance (see below for more information). The end-effector based robots operate essentially by “handshaking” with the patients. While there may be more uncertainty about the kinematics of patients’ limb motions, donning and doffing times are minimal with this class of devices. Furthermore, because the motors are typically mounted on the base of the end-effector robots and not carried on the robot joints, the devices can employ larger actuators with smaller transmission reduction ratios (including direct drive designs) leading to intrinsically lower impedance devices—a very important consideration to enhance robot performance and stability (see for example MIT-Manus). As a general rule, we typically consider exoskeletal designs when the human joint must rotate by more than 60 degrees (e.g., MIT’s wrist robot); otherwise we prefer end-effector designs.
Intrinsically High Impedance Versus Low Impedance Design
Exoskeletal or end-effector designs employ motors which have not only weight and mass to be accounted for in any design but also static and viscous friction. A comparison of the ratios of energy to weight for human muscle and for an electrical motor demonstrates the impressive competence of muscles. Muscles generate two to six times more energy for the same weight (typical electrical motors 50 W/kg and muscle 300 W/kg). In many robotic devices for which weight is a critical limitation (e.g., exoskeletal robots like MIT’s Anklebot), speed-reducing transmissions are employed to increase the torque output without excessive increase in weight.* An unintended consequence
of employing this reduction to boost the actuator torque is augmenting intrinsic impedance, especially friction. The perceived actuator static friction† increases by the reduction ratio, and the viscous friction§ by the square of the reduction ratio. To illustrate, by employing a gear reduction 200:1 to reduce actuator weight, we increase the perceived static friction by 200 and the viscous friction by 40,000. Therefore the resulting robot is likely to have an intrinsically high impedance; that is, if the robot is in the “off” state, it requires very high force to move it. Conversely, an end-effector, direct-drive robot that can employ larger actuators which are fixed at the base and are not mounted on the robot arms, has very low intrinsic and end-point friction and can easily be moved. The resulting robot is likely an intrinsically low impedance robot (e.g., MIT-Manus).
of employing this reduction to boost the actuator torque is augmenting intrinsic impedance, especially friction. The perceived actuator static friction† increases by the reduction ratio, and the viscous friction§ by the square of the reduction ratio. To illustrate, by employing a gear reduction 200:1 to reduce actuator weight, we increase the perceived static friction by 200 and the viscous friction by 40,000. Therefore the resulting robot is likely to have an intrinsically high impedance; that is, if the robot is in the “off” state, it requires very high force to move it. Conversely, an end-effector, direct-drive robot that can employ larger actuators which are fixed at the base and are not mounted on the robot arms, has very low intrinsic and end-point friction and can easily be moved. The resulting robot is likely an intrinsically low impedance robot (e.g., MIT-Manus).
For an interactive experience, the robot must allow even weak patients to express movement and the two distinct designs require very distinct control strategies to achieve this goal. An intrinsically high impedance robot must employ an aggressive control strategy to modify its behavior and reduce its inherent impedance to allow patients to express movement, no matter how weak. An intrinsically low impedance robot allows even weak patients to express movement, and simple control strategies can be employed to increase the robot’s inherent impedance whenever therapy requires guided or restricted movement. It is important to note that in practice, intrinsically high impedance robots suffer from a significant limitation: we cannot arbitrarily increase the gains of the controller to reduce the perceived impedance. Eventually, the robot stability, and therefore safety, is compromised.
Number of Degrees of Freedom
The optimum number of degrees of freedom is a difficult and critical question that requires considerable clinical insight supported by clinical results. The human body has many more degrees of freedom than any robotic device likely to be built. How many of those degrees of freedom should be actuated in a therapy robot? For instance, the human hand has 22 degrees of freedom and it is very unlikely that we will see any therapy robot in the near future that can interact in a controlled fashion with all of these degrees of freedom. This observation is valid for both upper and lower extremity. For the lower extremity, the ankle is a critical joint for gait. How many ankle degrees
of freedom do we need to actuate during gait rehabilitation? These questions can only be answered via exhaustive clinical trials based on a clinical understanding of the target user population. For example, MIT’s new hand module was designed to train cylinder grasp (10). We opted for such an apparent limited design from our clinical understanding and experience with a previous eight degrees-of-freedom hand robot: the user of MIT robotic systems is likely a severe-to-moderate stroke patient with significant hemiparesis of the hand and fingers. For these patients, we simplified the mechatronic design considerably by limiting our goal and providing a tool to facilitate the training of grasp. We made a compromise to get a relatively low cost, light, and compact module that can easily be integrated with the shoulder-and-elbow MIT-Manus or wrist robots. Another case of design compromise is the Lokomat, designed to actuate the hip and knee in the sagittal plane but with no actuation at the ankle (11). Both robots embody reasonable compromises based on the best available expert insight at the time, but can we do better? Ultimately, all these designs must undertake rigorous and exhaustive clinical trials to determine their adequacy. This is a critical aspect that clinicians must demand and technology developers cannot ignore and must be prepared to undertake. This critical requirement makes the deployment of rehabilitation robotics the antithesis of rapid-fire demonstrations observed in many other technology arenas. Therapy robots and control algorithms must pass through a set of painstaking exhaustive clinical trials to determine, as noted previously, what works, what does not, what works better, when to apply the robotic tools and under what protocol, and for whom.
of freedom do we need to actuate during gait rehabilitation? These questions can only be answered via exhaustive clinical trials based on a clinical understanding of the target user population. For example, MIT’s new hand module was designed to train cylinder grasp (10). We opted for such an apparent limited design from our clinical understanding and experience with a previous eight degrees-of-freedom hand robot: the user of MIT robotic systems is likely a severe-to-moderate stroke patient with significant hemiparesis of the hand and fingers. For these patients, we simplified the mechatronic design considerably by limiting our goal and providing a tool to facilitate the training of grasp. We made a compromise to get a relatively low cost, light, and compact module that can easily be integrated with the shoulder-and-elbow MIT-Manus or wrist robots. Another case of design compromise is the Lokomat, designed to actuate the hip and knee in the sagittal plane but with no actuation at the ankle (11). Both robots embody reasonable compromises based on the best available expert insight at the time, but can we do better? Ultimately, all these designs must undertake rigorous and exhaustive clinical trials to determine their adequacy. This is a critical aspect that clinicians must demand and technology developers cannot ignore and must be prepared to undertake. This critical requirement makes the deployment of rehabilitation robotics the antithesis of rapid-fire demonstrations observed in many other technology arenas. Therapy robots and control algorithms must pass through a set of painstaking exhaustive clinical trials to determine, as noted previously, what works, what does not, what works better, when to apply the robotic tools and under what protocol, and for whom.
CLINICAL RESULTS
Upper Extremity Rehabilitation and Stroke
As discussed earlier, the biggest hurdle faced in therapeutic robotics is not the technology per se but its clinical validation. But every challenge is also an opportunity: robots provide an ideal platform for objective, reproducible continuous measurement and control of therapy. For a glimpse at the clinical results, meta-analyses are an outstanding source for an overall picture and comparison basis; there are at least three recently published articles that include a range of devices with more to come (4, 5, 6, 7, 8). Herein, we will summarize some of our results for the shoulder-and-elbow transport of the arm collected during a long series of clinical experiments involving well over 400 participants. We will then conclude reviewing the main findings of two meta-analyses. We will not include in this chapter a discussion of results with upper extremity distal robots (12).
Subacute Rehabilitation
Volpe et al. reported the composite results of robotic therapy with 96 consecutive stroke inpatients admitted to Burke Rehabilitation Hospital in White Plains, NY (13). These patients were on average 2 weeks after an acute first stroke. All participants received conventional neurological rehabilitation during their participation in the study. The goal of the trial was to amass initial evidence to test whether robotic therapy had a measurable impact on recovery. Hence, we provided one group of patients with as much therapy as possible to address a fundamental question: does goal-oriented movement therapy have a positive effect on neuromotor recovery after stroke?
Patients were randomly assigned to either an experimental (robot-trained) or control (robot-exposure) group. Individuals in the robot-trained group were seen for five 1-hour sessions each week and participated in 25 sessions of sensorimotor robotic therapy for the paretic arm. Patients were asked to perform goal-directed, planar reaching tasks that emphasized shoulder and elbow movements with their paretic arm. MIT-MANUS’ low impedance guaranteed that the robot would not suppress any attempts to move. When a patient could not move or could not be deviated from the desired path or was unable to reach the target, the robot provided soft guidance and assistance dictated by an impedance controller (14). This robot action (which we dubbed “sensorimotor” therapy) was similar to the “hand-over-hand” assistance that a therapist often provides during conventional therapy. It is interesting to note that this form of “assistance as needed,” which has been a central feature of our approach from the outset (and a challenge for our robot designs), has recently been adopted and promoted by other groups (15,16).
Individuals assigned to the robot-exposure (control) group were asked to perform the same planar reaching tasks as the robot-therapy group for only 1 hour per week. However, the robot did not actively assist the patient’s movement attempts. When the subject was unable to reach toward a target, he or she could assist with the unimpaired arm or the technician in attendance could help complete the movement. The robot supported the weight of the limb while offering negligible impedance to motion. For this control group, the task, the visual display, the audio environment (e.g., noise from the motor amplifiers), and the therapy context (e.g., the novelty of a technology-based treatment) were all the same as for the experimental group, so this served as a form of “placebo” of robotic movement therapy.
The study was “double blinded” in that patients were not informed of their group assignment and therapists who evaluated their motor status did not know to which group patients belonged. Standard clinical evaluations included (a) the MRC motor power score for four shoulder and elbow movements (MP, maximum score = 20) and (b) the motor status score which is divided into two subscales, one for shoulder and elbow movements (MS-SE, maximum score = 40) and a second for wrist and hand abilities (MS-WH, maximum score = 42) (17, 18, 19, 20). The MS-SE and MS-WH have met standards for interrater reliability, significant intraclass correlation coefficients, and internal item consistency for inpatients (21).
Although the robot-exposure (control) and robot-treated (experimental) groups were comparable at admission, based on sensory and motor evaluation, on clinical and demographic scales, and the fact that both groups were inpatients in the same
stroke recovery unit and received the same standard care and therapy for comparable lengths of stay, the robot-trained group demonstrated significantly greater motor improvement (higher mean interval change ± SEM) than the control group on the MS-S/E and MP scores (Table 83-1). In fact, the robot-trained group improved twice as much as the control group by these measures. Notably, these gains were specific to motions of the shoulder and elbow, the focus of the robot therapy. There were no significant between-group differences in the mean change scores for wrist and hand function.
stroke recovery unit and received the same standard care and therapy for comparable lengths of stay, the robot-trained group demonstrated significantly greater motor improvement (higher mean interval change ± SEM) than the control group on the MS-S/E and MP scores (Table 83-1). In fact, the robot-trained group improved twice as much as the control group by these measures. Notably, these gains were specific to motions of the shoulder and elbow, the focus of the robot therapy. There were no significant between-group differences in the mean change scores for wrist and hand function.
TABLE 83.1 Burke Inpatient Studies (N = 96) Mean Interval Change in Impairment and Disability (Significance p < 0.05) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Our initial result supported our working model indicating that, to promote recovery, we need to harness the correct sequence of events much like the Hebbian rule for neural plasticity, which is colloquially summarized as “neurons that fire together wire together.” We believe that, in order to promote maximum recovery, events must occur in a proper sequence: the target is displayed, the patient attempts to move, movement then happens (either self-generated or assisted by the robot), and the sensory information comes back to the brain. Though this was a modest beginning, it provided unequivocal evidence that robotic therapy of the kind that might be delivered by a robot had a significant positive impact on recovery.
Follow-up
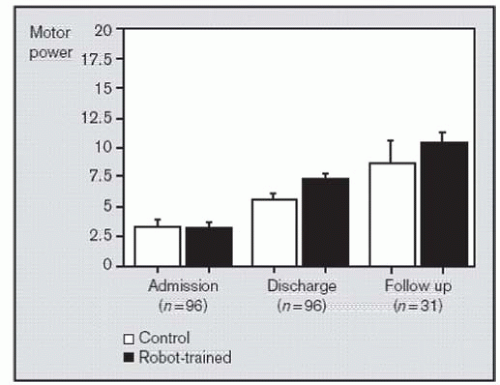
We were able to recall 31 of these 96 patients 3 years after discharge from the subacute hospital. The goal was to determine if the advantage conferred on the robot-trained group over the control group was sustained. It is possible that the short-term additional gains of the subacute phase were temporary and that, ultimately, patients in both the experimental and control groups would achieve the same clinical “plateau.” Such a result is of interest as it would suggest that robot-mediated therapy might contribute to the acceleration of recovery but not to the ultimate outcome. Figure 83-3 shows the motor power for these 31 patients. Results from this follow-up study demonstrated that the robot-mediated group maintained the advantage over the control group. Furthermore, we observed that both groups improved significantly from discharge of the subacute hospital to the 3-year follow-up. While we did not control any intervention during this period, this result showed further impairment reduction. Such a result is quite important because it contradicts epidemiological studies which suggested that, after 12 weeks poststroke onset, there is little opportunity for further impairment reduction (22,23). It also demonstrates that the clinical “plateau” has little support from biology and there is an opportunity to impact recovery in persons with chronic impairment due to stroke, perhaps many years after the injury.
Bolstered by this result showing the positive and measurable impact of robotic therapy on neurorecovery, we are systematically investigating the multitude of variables that may influence outcome to determine their independence, their interaction, and their actual impact on outcomes in acute, subacute, and chronic phases of stroke. Our goal is the “holy grail” of robotic therapy, to determine how best to customize the treatment protocol to meet each individual patient’s needs.
Continuous Passive Motion
Kwakkel has shown that a task-oriented training of high intensity is perhaps the most important aspect of a comprehensive stroke rehabilitation program (24). Volpe et al. tested whether intensity alone would suffice (25). To that end, they recruited subacute patients at the Burke Rehabilitation Hospital, White Plains, NY, and compared 25 minutes of additional conventional therapy with 25 minutes employing a CPM device (Continuous Passive Motion—Shoulder 600, Orthologic, Tempe, AZ), while patients’ attention was held by TV or social conversation. A CPM machine is an intrinsically high impedance device that employs position control and moves the patient’s shoulder or elbow passively. Patients cannot freely express any movement. Of note, there was no neurological advantage conferred on the CPM group. Patients in both groups showed comparable improvement in the neurological scales. This result reinforced our Hebbian view that, in order to improve outcomes, therapy must elicit the proper sequence of events and patients must participate actively and attempt to move. It is important to mention that while no neurological benefit was observed, the CPM group exhibited an apparent benefit in terms of shoulder stability and none of the patients in this group developed pain. Therefore, it became standard of care at Burke Rehabilitation Hospital to assign any stroke patient starting to show signs of shoulder pain to a daily session of movement therapy administered via the CPM machine.
Intensity-Matched Rehabilitation
The neurorehabilitation process is labor intensive, relying on therapy and evaluation procedures that are typically administered by a clinician working with a single patient. This one-on-one interaction characterizes much of the practice of clinical neurology. The repetitive nature of therapy makes it amenable to administration by properly designed robots. A robotic therapist can act as a modern, effective and novel tool, one that delivers a highly reproducible motor learning experience, quantitatively monitors and adapts to patients’ progress, and ensures consistency in planning a therapy program. Indeed, our research suggests that robotics can afford a much more intense experience than traditional therapy. For example, Kahn et al. noted that in a typical conventional therapy session, patients attempt to move their impaired arm circa 60 to 80 times (27). In a typical robot-mediated therapy session, patients must attempt to move 1,024 times. To confirm the importance of task-oriented training and intensity, we conducted a clinical trial including three groups of stroke patients: usual-care, robot-mediated therapy, and human-administered intensive-matched therapy. The last group was an attempt to match human-administered therapy and the intensity of typical robot-mediated sensorimotor therapy (1,024 attempts to move in each session). Though this latter form was somewhat artificial and impractical, results demonstrated that there were no significant differences in outcomes between robot-mediated and human-administered intensive-matched therapy groups; furthermore both groups improved far more than the usual-care group (28). These results further support Kwakkel’s assertion of the importance of a comprehensive rehabilitation program encompassing task-oriented movements of high intensity (24).
Motor Learning as a Model for Motor Recovery**
Given the apparent importance of high intensity and a patient’s active participation in therapy, we revised our robot control algorithm to test whether continuously challenging a patient would enhance recovery. The revised algorithm differs from our earlier sensorimotor therapy in three important ways (29).
First, during our earlier clinical trials, robotic therapy took the form of fixed, repetitive reaching exercises cued by a video display. An impedance controller with constant stiffness and damping made the therapy interactive: the force exerted by the robot varied continuously as a function of the deviation of the patient’s motion from a minimum-jerk trajectory of constant duration that connected the start position to the goal position. This system suited patients with limited motor ability for whom it provided assistance; however, it would also impede patients who moved faster than the nominal trajectory. Our revised algorithm used nonlinear impedance control to implement a “virtual slot” that extended between the start and goal positions and defined the appropriate coordination. Lateral deviation from the desired path was discouraged by the stiffness and damping of the slot sidewalls. Desired motion was assisted by moving the back wall of the slot along a minimum-jerk virtual trajectory so that the slot progressively “collapsed” to a “virtual spring” centered on the goal position. However, motion along the “virtual slot” (well aimed and faster than the nominal desired trajectory) was unimpeded.
A request for the subject to move was signaled by a target in the visual display changing color. If the patient failed to initiate movement within 2 seconds, the robot began to act (i.e., the back wall of the “virtual slot” closed on the goal position). This mode of triggering the robot encouraged even severely impaired patients to participate actively, rather than passively allowing the robot to drive the arm.
Second, the revised algorithm continuously monitored the patient’s performance. By combining records of the kinematics of actual patient motion and the kinetics of mechanical interaction between robot and patient, five performance measures (PMs) were computed: PM1 graded patients’ ability to initiate movement, PM2 measured movement range or extent, PM3 measured the power delivered by the patient to complete the movement, PM4 measured the smoothness of the movement, and PM5 measured aim or deviation from the straight line connecting the center and outbound target. We used these measures to adjust the parameters of the controller during a therapy session. For the first five cycles of movements to the eight goal positions and back to the center position, the time allotted for a movement (the duration of the nominal minimum-jerk trajectory) and the stiffness (impedance) of the
“virtual slot” sidewalls were adjusted approximately to match the patient’s current performance and need for guidance. This was important because patient performance typically declined between the end of one therapy session and the beginning of the next. For every subsequent five cycles of reaches to and from the eight goal positions, the controller parameters were adjusted based on the patient’s performance. The intent was to challenge the patient to improve.
“virtual slot” sidewalls were adjusted approximately to match the patient’s current performance and need for guidance. This was important because patient performance typically declined between the end of one therapy session and the beginning of the next. For every subsequent five cycles of reaches to and from the eight goal positions, the controller parameters were adjusted based on the patient’s performance. The intent was to challenge the patient to improve.
As patients aimed better, the stiffness of the “virtual slot” sidewalls was decreased (and vice versa). As patients moved faster, the time allotted for movement was decreased (and vice versa). Again, this was intended to encourage active participation of even the most impaired patients, yet continuously challenge patients as they recovered.
Third, to provide motivation, positive reinforcement, and knowledge of results, our revised algorithm provided specific, movement-related feedback in the form of a simple graphical display that consisted of five vertical bars to reflect recent patient performance. The height of each of the five bars was determined by the five PMs, expressed as a percentage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree