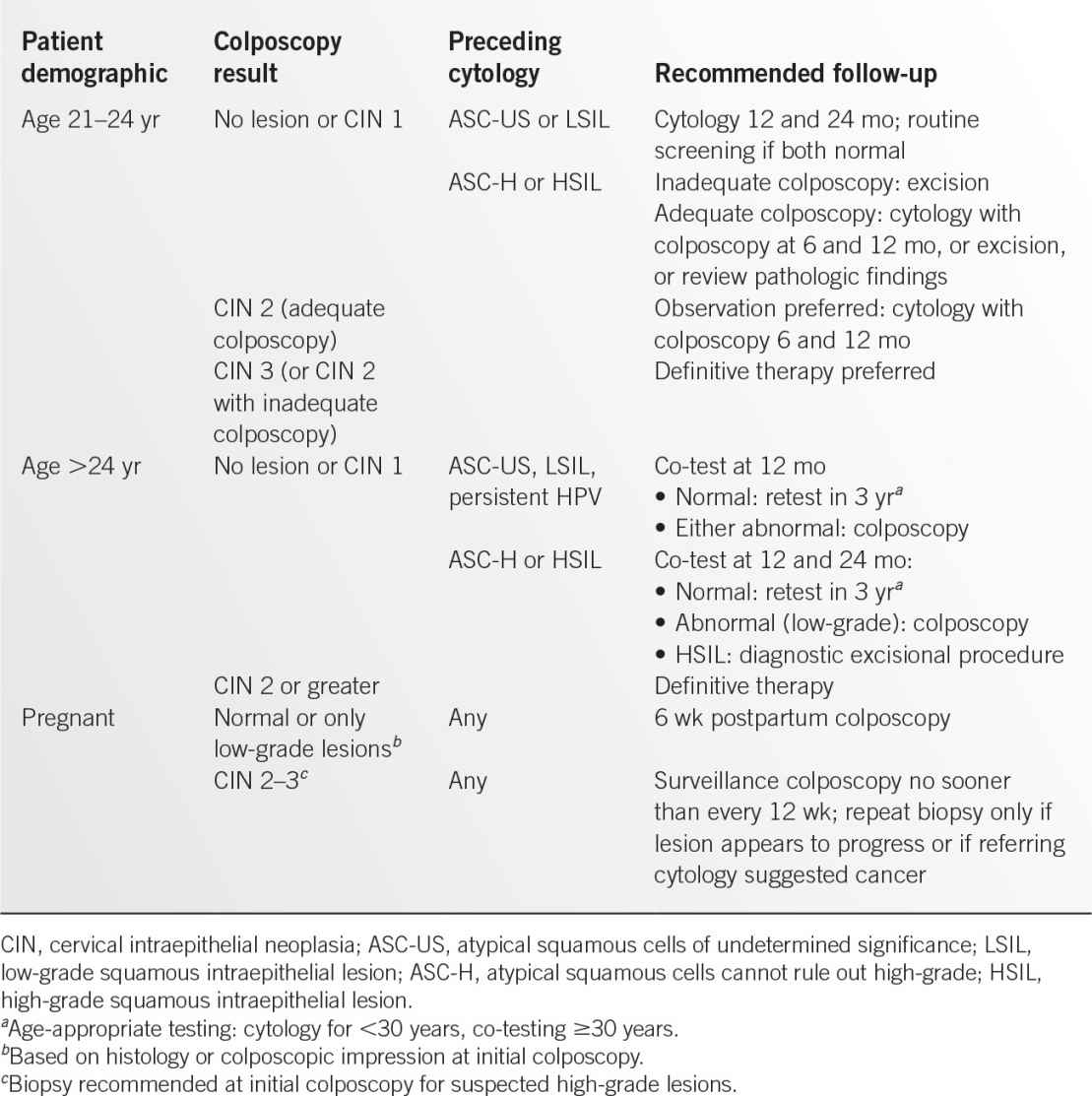
Figure 13.1-1. Vaginal smears of common vaginitis pathogens. A: Trichomonas vaginalis. B: Candida albicans. (A: Reproduced with permission from Sun T. Parasitic disorders: pathology, diagnosis, and management. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 1999; B: Reproduced with permission from Fleischer GR, Ludwig S. Baskin MN. Atlas of pediatric emergency medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.)
• 7 to 14 days topical therapy.
• Oral fluconazole (100, 150, or 200 mg dose) every third day for total three doses.
AND
• One of the above initial doses plus oral fluconazole weekly for 6 months.
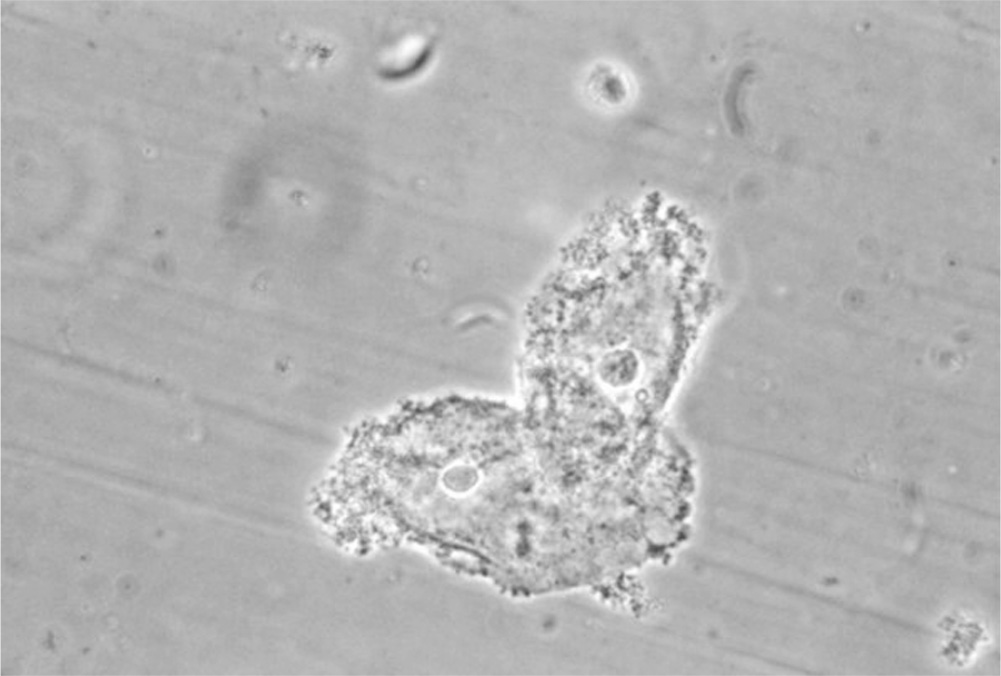
Figure 13.1-2. Clue cells. Clue cells are epithelial cells with clumps of bacteria clustered to their surface. These cells indicate the presence of bacterial vaginosis. (Courtesy M. Rein, Centers for Disease Control and Prevention Public Health Image Library.)
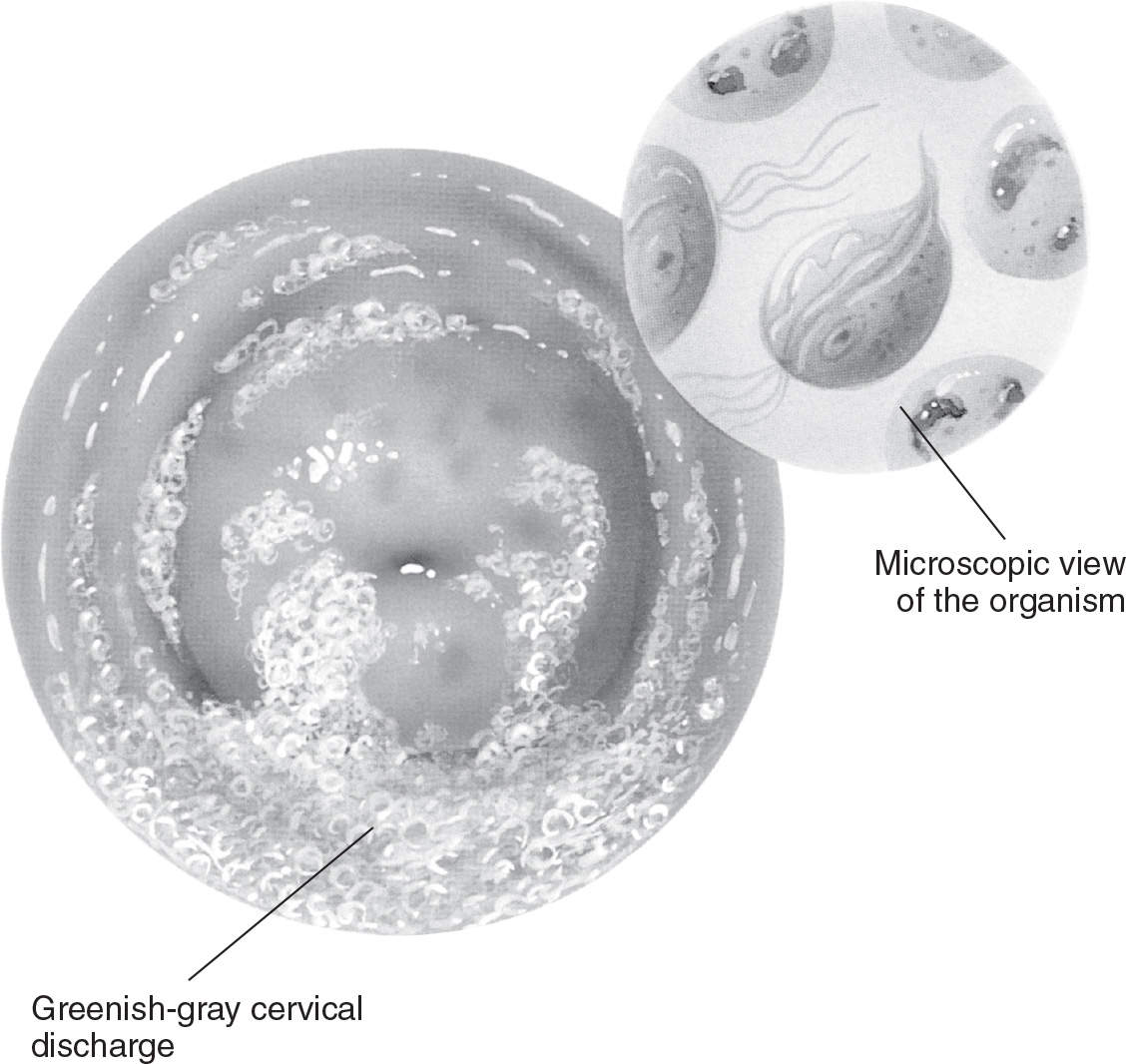
Figure 13.1-3. Trichomonal vaginitis. (From Anatomical Chart Company. Atlas of pathophysiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:313.)
Trichomoniasis
Trichomoniasis is a protozoal infection caused by the organism Trichomonas vaginalis. In contrast to BV and Candida infection, trichomoniasis is a sexually transmitted infection.
Diagnosis
Clinical Presentation
Women with T. vaginalis infections typically have a profuse, yellow/green, frothy vaginal discharge with an unpleasant odor (see Figure 13.1-3). Vulvovaginal irritation can also be present. Examination of the cervix may reveal punctate hemorrhages on the cervix and vagina (“strawberry cervix”). Patients may complain of postcoital bleeding. Many of these infections are asymptomatic.6
Laboratory Studies
• Microscopic examination with the presence of mobile, flagellated trichomonads on wet mount (see Figure 13.1-1A).
• The vaginal pH is elevated (>4.5).
• Pap tests are specific but not sensitive for infections with Trichomonas. Infections based on Pap test results should be treated, but a normal Pap test does not rule out infection.7
• Cultures or nucleic acid amplification testing (NAAT) should be sent when there is a suspicion for Trichomonas infection despite a normal wet mount result due to high false negative rates for this test.
Treatment and Prevention of Recurrence
Standard treatment is a single oral dose of metronidazole, and topical therapy has been shown to be less effective. Patients should be counseled on a possible disulfiram reaction if alcohol is consumed concurrently. Patients should be counseled on prevention of future episodes through the use of male or female condoms.7
Medications
• A single 2-g dose of metronidazole.
• Tinidazole in a single oral 2-g dose is also likely as effective. Topical metronidazole has been shown to be less efficacious than oral metronidazole.
• Sexual partners should be treated as well. Resistant or recurrent infections may require a higher dose or longer duration of treatment with metronidazole (i.e., 500-mg orally twice daily for 7 days); no other medication is currently available in the United States to treat trichomoniasis. Patients with an allergy to metronidazole should undergo desensitization treatment.
Atrophic Vaginitis
Atrophic vaginitis is caused by estrogen deficiency and usually occurs in postmenopausal women.1
Diagnosis
Clinical Presentation
Like other forms of vaginitis, atrophic vaginitis is often asymptomatic. Symptoms include vaginal soreness, burning, dyspareunia, and occasionally bleeding or spotting.
Physical Examination
The vaginal mucosa is thin, friable, and pale or erythematous if inflammation is present. It can appear dry, or patients may have a thin, watery discharge.
Laboratory Studies
• Vaginal pH is increased (5 to 7).
• Wet mount reveals parabasal cells (small, round epithelial cells with large nuclei) and polymorphonuclear leukocytes if inflammation is present.
Treatment
Patients can be reassured that mild symptoms are normal and do not require treatment. Dryness can be treated with vaginal lubricants. Topical or oral estrogen replacement is used to treat more bothersome symptoms of atrophic vaginitis, though patients should be counseled on long-term risks of endometrial carcinoma, particularly with higher doses of estrogen. Consider use of oral progesterone along with estrogen for long-term treatment.1
CERVICITIS
General Principles
Definition
Cervicitis refers to inflammation of the uterine cervix and is characterized by a purulent cervical discharge or a friable cervix on examination. White blood cells on wet mount or Gram stain are also common, although there is no standard number of white blood cells that confirms the diagnosis.8
Epidemiology
Chlamydia trachomatis and Neisseria gonorrhea are the most common identified causes of cervicitis; however, these infections may account for only 20% to 50% of women with cervicitis.5 Other common causes of cervicitis include infectious causes (Mycoplasma, Ureaplasma, bacterial vaginosis, herpes simplex, cytomegalovirus, Trichomonas, and adenovirus) and nonspecific inflammation.9 In some women, a cause will not be identified after diagnostic evaluation.8
Diagnosis
History
Vaginal discharge, postcoital bleeding, dyspareunia, and irregular vaginal bleeding are common symptoms of cervicitis. Many women are asymptomatic, and cervicitis may be found on an examination done for other reasons, such as Pap smear collection.3
Physical Examination
Physical examination commonly reveals mucopurulent discharge, cervical ectropion, and a friable cervix that continues to bleed after passage of a cotton swab through the cervical os. Women with cervicitis should also be examined for cervical motion tenderness, as this can indicate the presence of pelvic inflammatory disease.
Laboratory and Imaging
Women who present with cervicitis should initially be tested for gonorrhea, Chlamydia, BV, and Trichomonas. Testing for Chlamydia and gonorrhea is done with a NAAT and can be performed on urine, vaginal, or cervical samples. The diagnosis of BV is made by Amsel’s criteria (see Bacterial Vaginosis section). Trichomonal infection can be diagnosed on wet mount or through NAAT or culture.8
Differential Diagnosis
In addition to infectious causes, clinicians should consider the possibility of chemical irritants or cervical malignancy.8
Treatment
Medications
High-risk women, including younger women who are at risk due to their age, should receive empiric treatment for gonorrheal and chlamydial infections. When Trichomonas or BV is diagnosed in a woman with cervicitis, she should be treated. Empiric antibiotic treatment is not recommended for women who are at low risk or who have negative test results for specific infections.8 Please see the chapters on Chlamydia and Gonorrhea for more details about the treatment of these infections.
Patient Education
Gonorrhea, Chlamydia, and Trichomonas infections are spread by intercourse with an infected partner.3 Male and female condoms reduce the risk of transmission. It is important to recognize that a diagnosis of a sexually transmitted disease can be very stressful for a patient and her partner.
REFERENCES
1. Hainer B, Gibson M. Vaginitis: diagnosis and treatment. Am Fam Physician 2011;83:807–815.
2. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22.
3. Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines. MMWR 2010;59(No. RR-12):1–110.
4. Tibaldi C, Cappello N, Latino MA, et al. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin Microbiol Infect 2009;15:670–679.
5. Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical Infect Dis 2009;48(5):503–535.
6. Bachmann LH, Hobbs MM, Seña AC, et al. Trichomonas vaginalis genital infections: progress and challenges. Clinical Infect Dis 2011;53(Suppl 3):S160–S172.
7. Gülmezoglu AM, Azhar M. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2011;(5):CD000220. doi: 10.1002/14651858.CD000220.pub2.
8. Taylor SN, Lensing, S, Schwebke J, et al. Prevalence and treatment outcome of cervicitis of unknown etiology. Sex Transm Dis 2013;40:379–385.
9. Patel MA, Nyirjesy P. Role of mycoplasma and ureaplasma species in female lower genital tract infections. Curr Infect Dis Rep 2010;12:417–422.
| Dysmenorrhea and Premenstrual Syndrome |
DYSMENORRHEA
General Principles
Definition/Pathophysiology
Dysmenorrhea is cramping pain associated with menstruation. Primary (functional) dysmenorrhea is a painful paroxysmal syndrome that precedes or may accompany menses. It is not associated with pelvic pathology. Secondary dysmenorrhea is painful menses caused by pelvic disease. The pain is thought to be due to elevated levels of prostaglandin F2α. Prior to the onset of menses, cyclic progesterone withdrawal leads to the degradation of endometrial cell membranes. The cellular debris is converted to arachidonic acid, which is further metabolized by cyclo-oxygenase (COX) enzymes to form prostaglandins.1 The prostaglandins stimulate endometrial and uterine smooth muscle contractility and promote myometrial vasoconstriction. Ischemia develops, causing an angina equivalent in the uterus and results in pain. Other studies have also shown elevated leukotriene levels to be a contributing factor. Vasopressin was thought to be an aggravating agent, but vasopressin antagonists have shown no effect on the relief of menstrual pain.2
Epidemiology/Etiology
Primary dysmenorrhea is one of the most common gynecologic complaints, thought to affect from 50% to 90% of women of reproductive age. It is a leading cause of absenteeism for women under 30 years of age and the leading cause of school absences for adolescent women.3 Secondary (acquired) dysmenorrhea is pain that results from a pelvic abnormality. Endometriosis is the most common cause of secondary dysmenorrhea.4 Other possible etiologies include reproductive tract structural anomalies, adenomyosis, uterine tumors and leiomyomata, polyps, chronic salpingitis, pelvic inflammatory disease (PID), intrauterine device (IUD) use, cervical stenosis, irritable and inflammatory bowel syndromes, and urologic disorders. Several authors have correlated dysmenorrhea with smoking cigarettes, high intake of omega-6 fatty acids, nulliparity, depression, and stress. Causation has yet to be proved in rigorous controlled studies.
Diagnosis
Clinical Presentation
Primary dysmenorrhea usually appears within 12 months after menarche. It is characterized by symptom onset around the time of menses. Pain can be colicky or spasmodic and is usually felt in the lower abdomen, back, and thighs. Patients may also experience nausea, vomiting, diarrhea, headache, fatigue, and dizziness—prostaglandin-mediated symptoms. Menstrual flow may be heavier than normal. Symptoms are often worst on the first day of menses and then gradually resolves. Physical examination will be unrevealing. A pelvic examination is not initially required to make the diagnosis, especially in nonsexually active and virginal women. The diagnosis can generally be made on the basis of history alone. If an adolescent is sexually active, a pelvic examination should be performed due to the high risk of PID in this population.4 If the history and physical examination are inconsistent, or initial therapies are unsuccessful, further evaluation for secondary causes of dysmenorrhea should be pursued.
In secondary dysmenorrhea, onset is typically more than 2 years after menarche. Pain is not limited to the menstrual cycle. Pelvic and rectovaginal examinations should be performed if endometriosis is suspected. Physical examination may reveal adnexal masses, fixed uterus or reduced uterine mobility, and uterosacral nodularity in patients with endometriosis; mucopurulent cervical discharge in those with PID; and uterine asymmetry or enlargement in those with adenomyosis.4
Treatment
First-line therapy is either a nonsteroidal anti-inflammatory drug (NSAID; e.g., naproxen, ibuprofen, mefenamic acid-nonspecific COX inhibitors) or a specific COX-2 inhibitor (e.g., celecoxib). These medications act to decrease prostaglandin production, thereby decreasing both menstrual flow and prostaglandin-mediated pain. Medications should be taken 1 to 2 days before the onset of menses and continued on a fixed regimen for about 3 days.4 Because of the recent concerns about the safety of COX-2 inhibitors, the short duration of therapy for relieving primary dysmenorrhea, and the low cost of NSAIDs, it is prudent to recommend established NSAIDs with better long-term safety data as the preferred treatment.5 Topical heat has been shown to be more effective than placebo and may be as effective as NSAIDs in small studies.4 Other pharmacologic therapies for dysmenorrhea have included oral contraceptives (either traditional or extended cycle dosing), leuprolide, danazol, depot medroxyprogesterone, levonorgestrel-containing IUD, nifedipine, terbutaline, oral guaifenesin,6 magnesium, thiamine, aspirin, B12, vitamin E, fish oil supplements, and the Japanese herb Toki-shakayaku-san.5
Nontraditional modalities include acupuncture and acupressure, transcutaneous electrical nerve stimulation (TENS) unit therapy, and local application of unidirectional static magnets; however, there is limited and inconsistent evidence on their effectiveness.7 Surgery is considered the intervention of last resort. Surgical interventions include laparoscopic uterosacral nerve ablation, presacral neurectomy, and hysterectomy. The first-line treatment for dysmenorrhea caused by endometriosis is combined oral contraceptives.4 Treatment for secondary dysmenorrhea is directed to the specific underlying cause and referral to the appropriate specialist for further medical or surgical treatment should be made.5
Despite the great prevalence of dysmenorrhea, many patients will not report symptomatology unless the provider specifically inquires. Some chronic complications with inadequately treated primary dysmenorrhea include anxiety and depression. Infertility can become a complication with certain causes of secondary dysmenorrhea. Inquiry and intervention can result in significant improvement of quality of life for these women.
PREMENSTRUAL SYNDROME
General Principles
Definition
Premenstrual syndrome (PMS) is a poorly understood psychoendocrine condition characterized by an array of somatic, cognitive, affective, and behavioral disturbances that recur in cyclic fashion during the luteal phase of the menstrual cycle and resolve with the onset of menstruation. More than 150 symptoms have been documented, varying from mild to severe enough to disrupt normal activities and interpersonal relationships. Not all cycles are associated with PMS symptoms and not all premenstrual changes should be labeled PMS.
Pathophysiology/Etiology
PMS represents a biophysiologic, endocrine phenomenon. Altered levels of various hormones have been offered as the cause for premenstrual symptomatology, including estrogen, progesterone, prolactin, growth hormone, thyroid hormone, follicle-stimulating hormone, luteinizing hormone, antidiuretic hormone, insulin, prostaglandin, and cortisol. Studies have failed to confirm any of these as absolutely causative.8 However, women with PMSs are thought to have an altered response to normal gonadal steroids during the luteal phase and their effect on neurotransmitters such as serotonin and GABA in the CNS.9 Premenstrual symptomatology and behavior may also stem from social, psychologic, or cognitive dysfunction.
Diagnosis
Clinical Presentation
There is no typical presentation of PMS. Some of the more common physical symptoms include abdominal bloating and cramping, breast tenderness, fluid retention and weight gain, acne, cold sores, fatigue, and head and muscle aches. Emotional changes include anxiety, panic, depression, heightened aggressiveness, hostility, food craving, forgetfulness, insomnia, irritability, mood lability, poor concentration, tearfulness, and reduced coping skills. In 2000, the American College of Obstetrics and Gynecology (ACOG) published a practice bulletin of 10 PMS diagnostic criteria. According to ACOG, the diagnosis of PMS requires that a woman has one or more of the affective or somatic symptoms listed. Symptoms must occur during the 5 days before menses (late luteal phase) in each of the three prior menstrual cycles; be relieved within 4 days of the onset of menses; and not recur until at least cycle day 13. The symptoms must be bothersome to the patient. They must exist in the absence of any pharmacologic therapy, hormones, alcohol, or recreational drugs. Finally, all other psychiatric or medical disorders must be excluded.10 The American Psychological Association (APA) included severe PMS in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) as an axis I diagnosis called premenstrual dysphoric disorder (PMDD). The DSM-5 defines PMDD as a severe form of PMS in which symptoms of anger, irritability, and internal tension are prominent.11 A woman must experience five or more from a list of 11 symptoms to fit PMDD criteria. As with the diagnosis of PMS, the symptoms must be experienced only in the luteal phase; all other diagnoses must be excluded; and the patient must have experienced them for the majority of cycles within the past year.10
History/Physical/Laboratory
A detailed history must be obtained, including menstrual history and inquiries about alcohol, tobacco, and recreational drugs. A complete physical examination must be performed. The need for in-depth neurologic or psychologic evaluation may become apparent. No specific diagnostic test is available for detecting PMS/PMDD. Laboratory investigation should be tailored to the individual patient. For example, complete blood count and thyroid studies should be considered in patients with menorrhagia or chronic fatigue. Charting the menstrual cycle and documenting symptomatology must be done for two to three cycles. Patients write down the symptoms that trouble them most and rate the severity throughout the entire menstrual cycle. The Daily Record of Severity of Problems (DRSP) form, a self-administered questionnaire, is the most commonly used tool.11 Presence of luteal phase symptoms in at least two cycles, lack of follicular phase symptoms, and absence of other specific disease entities strongly suggest the diagnosis of PMS or PMDD.
Treatment
Nonpharmacologic
The clinician must individualize the treatment plan to maximize therapeutic response. Treatment should begin with a 2- to 3-month trial of lifestyle changes while the patient records symptoms. This is recommended in women with mild PMS that do not cause distress or socioeconomic dysfunction.12 Stress management strategies should be taught. Sufficient rest should be advocated. Regular aerobic exercise has been demonstrated to alleviate some PMS symptomatology, probably due to endogenous endorphin release. A well-balanced diet with adequate protein, fiber, and complex carbohydrates is essential for everyone’s good health. However, these recommendations have not been investigated in rigorous controlled studies. Caffeine, salt, excess sugar, tobacco, alcohol, and recreational drugs may worsen physical symptoms and emotional lability. Multivitamins, calcium, and magnesium supplements may be helpful. Pyridoxine (vitamin B6) may reduce fatigue, depression, and irritability in selected women. However, there is no convincing evidence that any of these are more effective than placebo and carry a potential for harm such as peripheral neuropathy with high-dose B6.12
Pharmacologic
If premenstrual complaints do not respond to the above and the presence of psychological disorders, substance abuse or hypothyroidism has been considered, medical therapy can be initiated. Symptom logs assist the clinician in tailoring treatment to individual needs. Prostaglandin inhibitors can relieve headaches, body aches, and dysmenorrhea. Spironolactone 25 to 50 mg bid during cycle days 14 to 28 may reduce fluid retention. Danazol and bromocriptine have been utilized in the past to reduce mastalgia. However, adverse side effects limit their usefulness.
Without menstrual cyclicity, PMS cannot occur. Oral contraceptives, depomedroxyprogesterone, levonorgestrel-containing IUDs, and gonadotropin-releasing hormone agonists have been tried with variable success. Oral contraceptive pills (OCPs) that contain the progestin drospirenone (an anti-mineralocorticoid and anti-androgen) are effective in reducing bloating and mood changes that accompany the placebo pill week. In 2012, the FDA stated the OCP-containing drospirenone may be associated with a higher risk of venous thromboembolism compared with levonorgestrel and other progestins.12 The recommendation is to assess each individual’s risk of Venous thromboembolism (VTE) prior to starting this medication in a new user. Multiple herbs have been utilized with variable success and safety in treating premenstrual symptoms. These include evening primrose oil, black current oil, chaste tree extract, black cohosh, wild yam root, dong quai, kava kava, and St. John’s wort. Interactions with other medications the patient might be taking must always be considered.13
Selective serotonin-reuptake inhibitors (SSRIs) are the first-line drugs for treating PMDD. Sertraline or fluoxetine are typically used first.12 They can be administered daily or only during the luteal phase (starting on cycle day 14). Treatment only during the luteal phase has fewer adverse side effects and is less expensive; however, it is important to ensure the patient is asymptomatic during the follicular phase or else she will be undertreated.12 If SSRIs are ineffective or patient still has residual symptoms despite SSRI therapy, one option is to augment treatment with low-dose alprazolam.12 Alprazolam is an anxiolytic with proven effectiveness for symptoms of premenstrual tension, anxiety, irritability, and hostility. However, its addictive potential makes it a second-line treatment. Buspirone is an effective anxiolytic that is not addictive.
PMS is a complex disorder of reproductive-aged women. Successful management requires continued communication and collaboration between patient and clinician.
REFERENCES
1. Harel Z. Cyclooxygenase-2 specific inhibitors in the treatment of dysmenorrhea. J Pediatr Adolesc Gynecol 2004;17:75–79.
2. Shushan A. Complications of menstruation and abnormal uterine bleeding. In: DeCherney A, Nathan L, Goodwin TM, et al., eds. Current diagnosis & treatment: obstetrics & gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:61–619.
3. Sultan C, Gaspari L, Paris F. Adolescent dysmenorrhea. In: Sultan C, ed. Pediatric and adolescent gynecology. Evidence-based clinical practice. 2nd ed. Basel: Karger; 2012:171–180.
4. Osayande A, Mehulic S. Diagnosis and initial management of dysmenorrhea. Am Fam Physician 2014;89:341–346.
5. Danakas G, Alvero R. Dysmenorrhea. In: Ferri FF, ed. Ferri’s clinical advisor 2014. Philadelphia, PA: Mosby; 2014:352.
6. Marsden JS, Strickland CD, Clements TL. Guaifenesin as a treatment for primary dysmenorrhea. J Am Board Fam Pract 2004;17:240–246.
7. Eccles NK. A randomized double-blinded, placebo-controlled pilot study to investigate the effectiveness of a static magnet to relieve dysmenorrhea. J Altern Complement Med 2005;11:681–687.
8. Winer SA, Rapkin AJ. Premenstrual disorders: prevalence, etiology and impact. J Reprod Med 2006;51:339–347.
9. Rapkin AJ, Akopians AL. Premenstrual Syndrome. https://www.clinicalkey.com/. Updated January 7, 2014. Accessed January 16, 2014.
10. Futterman LA, Rapkin AJ. Diagnosis of premenstrual disorders. J Reprod Med 2006;51:349–358.
11. Yonkers KA, Casper RF. Clinical manifestations and diagnosis of premenstrual syndrome and premenstrual dysphoric disorder. UpToDate. www.uptodate.com/home/index.html. Updated November 8, 2013. Accessed January 16, 2014.
12. Kasper RF, Yonkers, KA. Treatment of premenstrual syndrome and premenstrual dysphoric disorder. UpToDate. www.uptodate.com/home/index.html. Updated March 17, 2014. Accessed March 28, 2014.
13. Kaur G, Gonsalves L, Thacker HL. Premenstrual dysphoric disorder: a review for the treating practitioner. Cleve Clin J Med 2004;71:303–321.
| Abnormal Genital Bleeding in Women and Girls |
GENERAL PRINCIPLES
Definition
Abnormal genital bleeding is any blood loss from the vaginal or perineal area other than the individual menstrual pattern of flow of a premenopausal girl or woman, or the expected cyclic hormonal bleeding in a postmenopausal woman taking hormones.
Terminology
A revised terminology system for abnormal uterine bleeding (AUB) in non–gravida-reproducing women was introduced in 2011 by the International Federation of Gynecology and Obstetrics. Within this system, the etiologies of the symptoms of AUB are classified as “related to uterine structural abnormalities” and “unrelated to uterine structural abnormalities” and categorized by the acronym PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified).1
Anatomy
Bleeding coming from structures surrounding the vagina, rectum, and perineum must be differentiated from uterine bleeding originating from the cervical os. Pregnancy, structural pelvic pathology such as fibroids or polyps, benign and malignant tumors, anovulation, and coagulopathies are the leading causes.
Epidemiology
The incidence of genital bleeding from all causes is unclear, but AUB accounts for nearly one-third of all gynecologic visits, mostly at menarche or perimenopause. In postmenopausal women, an estimate of uterine bleeding in the first 12 months is 409 per 1,000 person-years, but only 42 per 1,000 person-years 3 years postmenopause.
Classification
• Nonuterine. The actual source of the bleeding may not be obvious. A laceration of the cervix, or of the vagina, especially if high in a fornix, may appear to be uterine bleeding. Urinary, rectal, or vulvar bleeding may be mistaken for vaginal bleeding.
• Vulvar or vaginal: infection, laceration, tumor, foreign body
• Extravaginal: perineal, urinary, rectal
• Systemic/medical: bleeding diathesis, thrombocytopenia; von Willebrand disease; liver, renal, endocrine disease
• Uterine. Age-grouping is important.
• Age: premenarche—any bleeding is abnormal
• Age: 13 to 40
![]() The clinician should consider pregnancy-related causes, especially ectopic pregnancy, even when bleeding is light or moderate, and even in young and perimenopausal women. Once pregnancy is ruled out, the diagnosis of dysfunctional uterine bleeding (DUB), although one of exclusion, does not require total certainty before reasonable treatment for anovulation is implemented.
The clinician should consider pregnancy-related causes, especially ectopic pregnancy, even when bleeding is light or moderate, and even in young and perimenopausal women. Once pregnancy is ruled out, the diagnosis of dysfunctional uterine bleeding (DUB), although one of exclusion, does not require total certainty before reasonable treatment for anovulation is implemented.
![]() Anovulatory bleeding (DUB) is common and may be treated as such without an exhaustive search for all other causes initially, as long as there is a normal medical history, pregnancy test, complete blood count (CBC), Pap smear when appropriate and a normal bimanual pelvic examination. Although not all uterine bleeding in this group will prove to be DUB, serious pathology in the face of normal findings is unlikely. If hormonal manipulation fails, a more complete workup can follow. Over age 35, an endometrial biopsy should be done.2
Anovulatory bleeding (DUB) is common and may be treated as such without an exhaustive search for all other causes initially, as long as there is a normal medical history, pregnancy test, complete blood count (CBC), Pap smear when appropriate and a normal bimanual pelvic examination. Although not all uterine bleeding in this group will prove to be DUB, serious pathology in the face of normal findings is unlikely. If hormonal manipulation fails, a more complete workup can follow. Over age 35, an endometrial biopsy should be done.2
• Age: 40 and older—higher index of suspicion of neoplasm. A very different scenario pertains to the peri- and postmenopausal woman, in whom bleeding must be investigated before anovulation can be assumed.
• Age: Postmenopausal—neoplasm until proven otherwise, unless known hormonal cause.
Etiology
• Pregnancy should be considered.
• Anovulation is a common cause (DUB, defined as uterine bleeding associated with anovulation, in the absence of other pathology):
• Physiologic: Adolescence (although 4% to 20% of adolescents have a coagulopathy underlying their abnormal bleeding), perimenopause (although abnormal bleeding must be considered neoplastic or hyperplastic until proven otherwise), lactation, and pregnancy.
• Pathologic: Hyperandrogenic anovulation (e.g., polycystic ovary syndrome, congenital adrenal hyperplasia, androgen-producing tumors), hypothalamic dysfunction (e.g., secondary to anorexia nervosa), hyperprolactinemia, hypothyroidism, primary pituitary disease, premature ovarian failure, and iatrogenic factors (e.g., secondary to radiation therapy or chemotherapy).
• Iatrogenic or treatment-induced:
![]() Estrogen withdrawal occurs after removal or irradiation of ovaries, or after giving and then withdrawing estrogen to a person without ovaries. (Midcycle bleeding can be due to preovulation drop in estrogen.)
Estrogen withdrawal occurs after removal or irradiation of ovaries, or after giving and then withdrawing estrogen to a person without ovaries. (Midcycle bleeding can be due to preovulation drop in estrogen.)
![]() Estrogen breakthrough is due to stimulation of endometrium from unopposed low- or high-level estrogen. (Low-dose estrogen produces intermittent light spotting; high-dose estrogen yields amenorrhea followed by profuse bleeding. Cyclic progesterone corrects this.)
Estrogen breakthrough is due to stimulation of endometrium from unopposed low- or high-level estrogen. (Low-dose estrogen produces intermittent light spotting; high-dose estrogen yields amenorrhea followed by profuse bleeding. Cyclic progesterone corrects this.)
![]() Progestin withdrawal occurs only if there has been prior estrogen priming.
Progestin withdrawal occurs only if there has been prior estrogen priming.
![]() Progestin breakthrough can occur when endometrium becomes so atrophic that lack of estrogen effect yields too little and too ragged a lining for synchronous cellular events. (Estrogen replacement therapy can restore responsiveness. This occurs after months on oral contraceptives (OCs) or depoprogesterone. Adding estrogen for a week usually corrects the problem.)
Progestin breakthrough can occur when endometrium becomes so atrophic that lack of estrogen effect yields too little and too ragged a lining for synchronous cellular events. (Estrogen replacement therapy can restore responsiveness. This occurs after months on oral contraceptives (OCs) or depoprogesterone. Adding estrogen for a week usually corrects the problem.)
• Noncyclic uterine bleeding (non-DUB)
• Uterine leiomyoma, leiomyosarcoma, endometrial polyp(s)
• Endometrial hyperplasia or carcinoma
• Cervical or vaginal neoplasia
• Endometritis, adenomyosis
• Bleeding associated with pregnancy (threatened or incomplete abortion, trophoblastic disease, ectopic pregnancy)
• Bleeding associated with the puerperium (retained products of conception, placental polyps, subinvolution of the uterus)
• Coagulopathies (von Willebrand disease, platelet abnormalities, thrombocytopenic purpura)
• Iatrogenic causes, medications, and devices—intrauterine devices (IUDs), diaphragms, pessaries
• Systemic diseases (liver, renal, thyroid, other endocrine)
• Infection, laceration or contusion, tumor, foreign body
• Perineal, rectal, urinary disease
• Systemic medical causes include bleeding diatheses, especially thrombocytopenia; von Willebrand disease; and liver, renal, endocrine disease
• Ovulatory excessive bleeding2
DIAGNOSIS/TREATMENT
Clinical Presentation
Abnormal genital bleeding can range from urgent or emergent bleeding to mild irregular bleeding in between menstrual cycle. Most common cause is pregnancy or its complications.
Assessment/History
When taking a detailed history, several questions must be answered, including pregnancy status, reproductive status, and the source of bleeding. This will help determine differential diagnosis and disposition of the patient. History should include relevant medical history, menstrual history, sexual history, contraceptive history, family history of bleeding disorders and thyroid diseases, and risk factors for endometrial cancer.
Severity
• Rate of flow. How heavy is the bleeding? Pad counts are unreliable because of differences of absorbency but may give a rough estimate. The patient’s own opinion is probably more valid. When did it start? Is the blood bright red or dark, with or without clots? If it is heavier than she has ever seen it, if it is flowing, if brighter red than menstrual blood, and if there are clots or pieces of tissue, there might be significant hemorrhage and the patient should not wait even a short time. If she cannot get to the office immediately, she should go to the nearest emergency room, by ambulance or 911. She should be instructed to retrieve any tissue passed for the purpose of analysis.
• Amount of flow. A rough estimate of blood loss can be made by asking how much more bleeding than a usual period she has had since onset. (Normal menstrual blood loss is 20 to 80 cc.)2
Associated Symptoms
Has there been fever, dizziness, abdominal pain, and diarrhea? Any of these could signal associated pelvic infection or abscess, shock, severe loss of blood volume, dehydration, other intra-abdominal pathologic process, or bleeding tendency.
Physical Examination
Check vital signs; do abdominal, perineal, vaginal, pelvic, and rectal examination. The examination must meticulously pinpoint the exact source, which may not be obvious. A physical examination with good exposure for the speculum examination, and optimal palpation of pelvic organs using bimanual and rectovaginal techniques, is crucial to finding serious and treatable pathology. Obesity and hirsutism should be noted. An estimate of prior hormonal influences should be made in an attempt to classify the type of anovulation.2
Basic Laboratory Tests
Initial tests should include pregnancy test (β-human chorionic gonadotropin) and CBC with platelets and differential. Additional testing based on history and physical examination may include endocrine, thyroid function tests, prolactin level, androgen level, follicle-stimulating hormone/luteinizing hormone, estrogen levels, and coagulation studies.2,3
Examination Findings
Examination findings may suggest other studies, such as endometrial biopsy or colposcopy, hysteroscopy, pelvic and/or endovaginal sonography (transvaginal scan [TVS]), hysterosalpingography, and saline infusion hysterosonography (saline infusion sonography [SIS]), usually performed by radiologists or gynecologists. Most procedures carry known risks, benefits, and advantages, and are chosen based on individual needs of the specific patient and circumstance.
Ultrasonography
Pelvic ultrasound is the first-line imaging study in women with AUB. Transvaginal examination should be performed, unless there is a reason not to do a vaginal examination. Ultrasound is effective at characterizing uterine and adnexal lesions. If it is performed in a postmenopausal woman, an endometrial biopsy is mandatory when the endometrial thickness is greater than 4 mm. In premenopausal women, endometrial findings on ultrasound is not a useful test since major variation during normal menstrual cycle.4
Endometrial Biopsy
After pregnancy has been excluded, endometrial biopsy should be performed to exclude endometrial neoplasia or endometritis. Endometrial biopsy should be performed in all women age 45 years or older with AUB, women below age 45 years with persistent AUB who failed medical management, those with a history of unopposed estrogen exposure, or those at high risk for endometrial cancers.3
Management
Treatment for specific pathology depends on the underlying cause and may be managed by the family doctor or may require referral to a gynecologist, endocrinologist, or gynecologic oncologist. Acute, heavy bleeding requires close observation, accurate determination of the source and likely cause, and immediate therapy. Hospitalization, hydration, and transfusion may be required.
Medical Management
In the hemodynamically unstable patient, fluid resuscitation and blood replacement are the first priority. Therapeutic options are intravenous high-dose estrogen, intrauterine tamponade, uterine curettage, uterine artery embolization, and hysterectomy. High-dose intravenous estrogen (Permarin) 25 mg is given every 4 to 6 hours for 24 hours. If bleeding does not subside after 8 hours, other treatments should be used.5 Antiemetics should be given for nausea.
In patients that are hemodynamically stable with severe uterine bleeding patient, first-line therapy is high-dose oral estrogen (Premarin) 2.5 mg four times per day until the bleeding subsides or is minimal. After oral estrogen has been discontinued, oral progestin (medroxyprogesterone acetate) 10 mg per day for 10 days should be initiated.6 Other options include high-dose oral monophasic contraceptives (containing 35 mcg ethinyl estradiol) three times a day for 7 days or medroxyprogesterone acetate 20 mg orally three times a day for 7 days.6 Tranexamic acid, an antifibrinolytic drug, dosed at 1.3 g three times a day for 5 days is another option that may be used for women with contraindications to hormonal therapy.7 For all patients, the contraindications to these therapies need to be considered before administration. In patients that are experiencing mild to moderate uterine bleeding, first-line therapy is usually oral estrogen–progestin contraceptives. Oral estrogen–progestin should contain 30 to 35 mcg ethinyl estradiol to reduce bleeding.6 For women with contraindications to estrogen therapy, levonorgestrel-releasing IUD is a reasonable choice.
Once the acute episode of bleeding has been controlled, multiple treatment options are available for long-term treatment of chronic AUB. These included OCs, progestin therapy (oral or intramuscular), levonorgestrel intrauterine system, tranexamic acid, and nonsteroidal anti-inflammatory drugs. Duration of treatment depends on circumstances of bleeding, fertility or contraceptive needs, and the age of the patient. Anemic patients should receive iron supplementation.
Patients with known or suspected bleeding disorders may respond to hormonal and nonhormonal management. Consultation with hematologist is recommended. Patients with von Willebrand disease may respond to desmopressin. For severe bleeding, recombinant factor VIII and von Willebrand factor may be required to control bleeding. Avoid nonsteroidal anti-inflammatory drugs for patient with bleeding or platelet dysfunction.8
Surgical Management
The need for surgical treatment is based on the clinical stability of the patient, the severity of bleeding, contraindications to medical management, the patient’s lack of response to medical management, and the underlying medical condition of the patient. Surgical options include dilation and curettage (D&C), endometrial ablation, uterine artery embolization, and hysterectomy. The choice of surgical modality is based on medical factors plus the patient’s desire for future fertility.
Special Considerations
• Structural or anatomical causes concurrent with DUB. Fibroids, especially when large, can degenerate and be the primary source of bleeding. However, fibroids are common and their presence, especially if small, does not mean that they are the source of the bleeding. DUB may still be the primary diagnosis, as may cancer. DUB or infection can occur with an IUD in place, which may be retained if treatment of the underlying cause is successful.
• Postmenopausal bleeding
• For the woman not on hormones, the decision is clear. She needs a thorough investigation of the cause of the bleeding, including endometrial sampling, to rule out endometrial cancer. Hysteroscopy, TVS, or SIS may be needed.
• For the woman on hormones, an individual decision must be made based on her prior problems and her hormone regimen.
![]() Unopposed estrogen should not be used for long-term chronic AUB. If patients are identified as taking unopposed estrogen, they must have endometrial biopsy.
Unopposed estrogen should not be used for long-term chronic AUB. If patients are identified as taking unopposed estrogen, they must have endometrial biopsy.
![]() Although continuous or monthly progesterone is protective, endometrial cancer risk is not entirely removed by its addition to the estrogen regimen. Cancer must be ruled out by endometrial biopsy in the face of persistent bleeding.
Although continuous or monthly progesterone is protective, endometrial cancer risk is not entirely removed by its addition to the estrogen regimen. Cancer must be ruled out by endometrial biopsy in the face of persistent bleeding.
![]() Patients taking progesterone less than monthly should have endometrial sampling if bleeding is off schedule. It is reasonable to obtain an endometrial sample without prior ultrasonographic examination; the procedure is simple and yields definitive tissue, although it can also miss areas.
Patients taking progesterone less than monthly should have endometrial sampling if bleeding is off schedule. It is reasonable to obtain an endometrial sample without prior ultrasonographic examination; the procedure is simple and yields definitive tissue, although it can also miss areas.
![]() Patients taking tamoxifen are at higher risk for endometrial cancer; those taking raloxifene are at lower risk.9
Patients taking tamoxifen are at higher risk for endometrial cancer; those taking raloxifene are at lower risk.9
• Perimenopause. Although hormonal therapy remains controversial, it is now common in perimenopause. Decision-making must take into account the special circumstances of the bleeding. In some cases, one can treat the hormonal transition as DUB or hormonal bleeding, before doing more testing. In others, endometrial sampling and/or imaging is advised. It is better to err on the side of sampling/imaging.
• Cervical stenosis. If endometrial biopsy is impossible, an ultrasound scan with an acceptable endometrial stripe (less than 4 to 5 mm) may suggest that therapy for DUB is reasonable. If the endometrial stripe is greater than 8 mm, referral to a gynecologist (with probability of D&C) is indicated.
REFERENCES
1. Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravida women of reproductive age. Int J Gynaecol Obstet 2011;113:3–13.
2. Sweet MG, Schmidt-Dalton TA, Weiss PM. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician 2012;85(1):35–43.
3. Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197–206.
4. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 440. The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol 2009;114:409–411.
5. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol 2013;121:891–896.
6. Munro MG, Mainor N, Basu R, et al. Oral medroxyprogesterone acetate and combination oral contraceptives for acute uterine bleeding: a randomized controlled trial. Obstet Gynecol 2006;108:924–929.
7. James AH, Kouides PA, Abdul-Kadir R, et al. Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol 2011;158:124–34.
8. American College of Obstetricians and Gynecologists Committee on Adolescent Health Care; American College of Obstetricians and Gynecologists Committee Gynecologic Practice. ACOG committee opinion no. 451. Von Willebrand disease in women. Obstet Gynecol 2009;114:1439–1443.
9. Speroff L, Fritz M. Postmenopausal hormone therapy. In: Fritz MD, Speroff L, eds. Clinical gynecologic endocrinology and infertility. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011:749–854.
| Pap Smear Evaluation for Cervical Cancer |
GENERAL PRINCIPLES
Definition
The Pap smear is the primary detection tool for cervical cancer. Since its widespread acceptance following publication of Papanicolaou and Traut’s paper in 1943,1 developed countries using the Pap smear as screening have had dramatic drops in rates of cervical cancer. Cervical cancer resulted in 3,939 deaths in the United States in 2010.2
Pathophysiology
The cervix is the inferior extension of the uterus. The vaginal portion is covered by squamous epithelium peripherally and centrally to a point referred to as the squamocolumnar junction (SCJ), where columnar (glandular) epithelium progresses from that point inward through the cervical os and into the body of the uterus. At birth, the SCJ is effaced more laterally on the vaginal portion of the cervix compared to the finding in a nonpregnant adult. A process known as squamous metaplasia results in the change from columnar to squamous epithelium between the original SCJ and the “new” SCJ. This area is referred to as the transformation zone (TZ). Virtually all cervical dysplasia and cancer occurs within the limits of the TZ, as it is the most “mitotically active” region of the cervix.
Risk factors for development of cervical cancer include human papillomavirus (HPV) exposure, early age of initiation of sexual activity, multiple sexual partners, cigarette smoking, and in utero diethylstilbestrol (DES) exposure. There is a dramatic difference (approximately threefold) between more and less developed countries in the incidence of cancer, primarily due to the availability of screening via the Pap smear.3
It is now well established that HPV is the causative agent of cervical cancer. This sexually transmitted virus exists in over 100 “strains,” most of which are not felt to be oncogenic. The known “high-risk” HPV subtypes are strains 16, 18, and approximately 10 others. Types 16 and 18 are responsible for approximately 70% of cervical cancers. Types 6, 11, and others are implicated as causes of condyloma. The other known risk factors for cervical cancer accelerate the oncogenicity of the high-risk subtypes; in addition, acquiring a new partner who exposes the patient to new low-risk strains accelerates the risk of cancer in a patient who already has high-risk strains of HPV.3
HPV is transmitted when infected genital epithelial cells desquamate during intercourse and bind to basal keratinocytes in areas of microtrauma on the sexual partner. The immune response of the host is usually inadequate to kill the virus because the virus does not kill the infected cells. It is believed that 20% of infections are handled through humoral immunity, and approximately 20% of infected patients have persistent infection despite therapy. The majority of patients, therefore, respond to therapy for warts or dysplasia with a lasting clinical remission. Patients with persistent infection may progress to low-grade disease, high-grade (cervical intraepithelial neoplasia, or CIN) disease, or invasive cancer. Severe dysplasia may still take up to 7 years to progress to invasive cervical cancer.3
DIAGNOSIS
Clinical Presentation
The great majority of patients who present with cervical cancer have had no screening for several years. The precursor conditions are imminently treatable when caught in any stage prior to invasive disease. Other than presenting for routine annual screening, patients may present with intermenstrual or postcoital bleeding, or have been referred for colposcopy because of a gross lesion.
History
Important aspects of the history, in addition to elucidation of risk factors mentioned above, include documentation of last menstrual period (LMP), any history of prior abnormal Pap or HPV testing, and history of prior treatment(s) for cervical disease. In older patients who may be menopausal, current or former hormone replacement therapy should be documented.4
Physical Examination
Screening should begin at age 21 with cytology alone every 3 years. Women less than age 21 should not be screened regardless of the time of first sexual intercourse. Women age 30 to 65 should be screened with cytology and HPV contesting every 5 years or with cytology alone every 3 years. It is not necessary to screen women after age 65 with prior negative screening. Direct visualization of the cervix with a speculum examination is necessary for collection of a sample for Pap smear. Patients should avoid douching or intercourse for 24 hours prior to the procedure. Metal speculums should be warmed with water; the new thin-layer preps do not require avoidance of lubrication for insertion of the speculum, but most pathologists still feel it should be avoided when possible. Note should be taken of any bleeding that is spontaneous or induced by contact with the instruments used to collect the sample. Any lesions, such as leukoplakia and Nabothian cysts, should be documented. A thorough bimanual examination is a standard part of an annual evaluation, with attention to size and position of the uterus and any nodularity or masses noted in the parauterine and adnexal regions.4
Laboratory Studies
Thin-layer (“thin-prep”) cytology is now the standard collection technique for Pap smear. This specimen has the same sensitivity as older, conventional smears and also allows for detection of HPV in the same sample. The “broom” is centered over the cervical os and twirled with pressure against the cervix two full rotations, and then deposited into the collection medium. In patients whom have had hysterectomy for advanced cervical dysplasia/cancer, the vaginal cuff is sampled. It is standard care now to request reflex testing for high-risk HPV subtypes if “atypical squamous cells of undetermined significance” (ASCUS) is found in the sample (discussed further under Pathologic Findings).4
Pathologic Findings
Adequacy of the collection is noted on the pathologist’s report; if endocervical cells are not detected in a patient who has a cervix, whether or not it should be recollected depends on age and HPV status. If the specimen is reported as inadequate, it should be recollected in 2 to 4 months. The Bethesda system adopted in 2001 includes the following:
• Statement of adequacy of the sample
• A general categorization of the findings, benign or malignant
• Descriptive diagnoses that may include benign changes (reactive, or secondary to infection). Epithelial changes may be squamous or glandular. The squamous components include ASCUS; HPV changes including “koilocytotic atypia,” low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and squamous cell cancer (SCC). The glandular components include atypical glandular cells of undetermined significance (AGUS), adenocarcinoma in situ (AIS), and adenocarcinoma.5
TREATMENT
Nonoperative
Patients with Pap smears showing ASCUS with high-risk HPV subtypes present, and with LSIL, HSIL, or SCC need colposcopic evaluation. ASCUS without high-risk HPV present may repeat co-testing in 3 years. Those with cytology negative but HPV-positive test results can be followed with repeat co-testing in 12 months or HPV DNA typing may be done and those with HPV 16 or 18 should undergo colposcopy. Any other subtypes may repeat co-testing in 1 year. Patients with AGUS should undergo colposcopy, and endometrial biopsy if appropriate. Adenocarcinoma, in situ or otherwise should be referred to a gynecologic oncologist, as the source of the abnormal cells could be endometrial, tubal, ovarian, or even from non-gynecological abdominal metastasis.4
Operative
After colposcopy with appropriate biopsies and endocervical curettage, patients are managed on the basis of American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines. Recommendations may include a diagnostic excisional procedure.4
Counseling
With the ease and efficacy of modern techniques for the management of Pap smear abnormalities, virtually all patients can be assured that they will never develop cervical cancer if they maintain appropriate follow-up visits for any abnormalities. Counseling about the risk factors for accelerating their risk of cancer (new contacts, smoking) is appropriate.4
Follow-Up
Telephone follow-up with abnormal Pap smear results is usually appropriate. Normal results are often notified by mail. Patients should be specifically told when their next screening is due. Guidelines for follow-up Pap, HPV testing, and/or colposcopy are well established by the ASCCP.4
Complications
Other than transient discomfort and occasional mild spotting, collection of Pap smears is not associated with any complications.
REFERENCES
1. Papanicolaou GN, Traut HF. Diagnosis of uterine cancer by the vaginal smear. Yale J Biol Med 1943;15:924.
2. U.S. Cancer statistics Working Group. United States cancer statistics: 1999–2010 incidence and mortality web-based report. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention and National Cancer Institute; 2013. www.cdc.gov/uscs. Accessed July 19, 2014.
3. Feldman S, Sirovich BE, Goodman A. Screening for cervical cancer: Rationale and recommendations. Uptodate. uptodate.com. Accessed July 19, 2014.
4. Saslow DS, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62(3):147–172.
5. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System terminology for reporting results of cervical cytology. JAMA 2002;287:2114–2119.
|
GENERAL PRINCIPLES
Definition
Acute pelvic inflammatory disease (PID) is an ascending infection of the female genital tract involving the uterus, fallopian tubes, ovaries, and adjacent pelvic structures.
Epidemiology
• More than 750,000 American women are diagnosed and treated for acute PID each year.1
• Direct medical costs of PID are estimated at $1.5 billion per year.2
Pathophysiology
• PID arises from the ascent of microorganisms from the vagina and cervix into the upper female genital tract.
• Although PID commonly stems from a cervicitis caused by Neisseria gonorrhoeae or Chlamydia trachomatis, there is evidence that an imbalance in the vaginal ecosystem, such as that seen in bacterial vaginosis, may also play a role in initiating the ascending infection.3
Etiology
• Microorganisms recovered from the upper genital tract of women with PID include C. trachomatis, N. gonorrhoeae, and anaerobic and aerobic bacteria of the endogenous vaginal flora, including Prevotella species, Peptostreptococcus, aerobic Streptococcus, Gardnerella vaginalis, Haemophilus influenzae, and enteric Gram-negative rods.4
• Epidemiologic risk factors that identify a patient at increased risk for acute PID include age less than 25 years, sexarche prior to age 16 years, multiple sexual partners, history of a sexually transmitted disease (including PID), the postinsertion period in intrauterine device (IUD) users, vaginal douching, and the presence of bacterial vaginosis.2,5,6
DIAGNOSIS
• As a result of the difficulty of diagnosis and its serious consequences if left untreated, guidelines for its diagnosis developed by the U.S. Centers for Disease Control and Prevention (CDC) reflect a lowering of the diagnostic threshold.7
• Once competing diagnoses are adequately excluded in a woman at risk for sexually transmitted diseases (STDs), the CDC recommends that a provisional diagnosis of PID be made and a therapeutic trial of antibiotics be initiated in patients who meet one or more of the following criteria on pelvic examination:
• Cervical motion tenderness
• Uterine tenderness
• Adnexal tenderness
• Although not required, corroborating diagnostic laboratory, imaging, and surgical procedures should be sought in patients with an unclear diagnosis, no evidence of lower-genital-tract inflammation, and severe symptoms, or who fail to respond to therapy.3,7
Clinical Presentation
PID can present with a wide spectrum of nonspecific clinical symptoms and signs, ranging in degree from mild to severe.
History
• Lower abdominal pain, usually described as constant and dull, of less than 14-days duration is the most common complaint reported by patients with acute PID.
• Other manifestations include abnormal vaginal discharge, abnormal vaginal bleeding, gastrointestinal upset, and dysuria.
• Right upper quadrant pain secondary to perihepatitis (Fitz–Hugh–Curtis syndrome) is seen in up to 10% to 15% of patients.8
Physical Examination
• Cervical motion tenderness and adnexal tenderness (unilateral in up to 20% of cases) are the physical findings most frequently elicited in patients with PID.
• Rebound tenderness is present in two thirds of patients, and an adnexal mass or fullness in 16% to 49% of patients.
• Although a temperature of 38.3°C or higher supports the diagnosis, it is important to be aware that fever is a variable finding present in 24% to 60% of patients.9
Laboratory Studies
• White blood count. A leukocytosis is present only 60% of the time.
• Erythrocyte sedimentation rate (ESR). Although classically elevated in PID, the ESR is normal (less than 15 mm per hour) in 25% of patients.
• C-reactive protein. An elevated C-reactive protein (CRP) >10 mg per dL has been found in up to 93% of patients with PID.10
• Examination of the male partner for the presence of urethritis can be a source of confirmatory evidence for the diagnosis of PID.
• A sensitive pregnancy test should be routinely obtained in all patients with suspected PID because of the great difficulty encountered in clinically differentiating patients with PID from those with ectopic pregnancy.11
• The finding of mucopurulent cervicitis or evidence of white cells on microscopic examination of a saline preparation of vaginal fluid is seen in the great majority of patients with PID. If both are absent, the diagnosis of PID is unlikely and alternative causes of pain should be considered.7,10,12
• Laboratory documentation of a cervical infection with N. gonorrhoeae or C. trachomatis corroborates the diagnosis of PID.7
• Cultures have traditionally been regarded as the gold standard.
• Nucleic acid amplification tests for the detection of C. trachomatis and N. gonorrhoeae are preferred.
Imaging
• Transvaginal pelvic ultrasonography. Sonographic findings supportive of the diagnosis include:
• Thickened, fluid-filled fallopian tubes
• Fluid in the cul-de-sac
• A complex, multiloculated adnexal mass
• Hyperemia on power Doppler transvaginal sonography3,13
• Magnetic resonance imaging (MRI). MRI findings that support the diagnosis of PID include:
• Fluid-filled tubes
• Thickened tube walls with a dilated lumen
• An ill-defined adnexal mass with thickened walls containing fluid14,15
Surgical Diagnostic Procedures
• Endometrial biopsy. The histopathologic finding of neutrophil and plasma cell infiltration in the endometrial stroma obtained on biopsy confirms the diagnosis of PID.10
• Diagnostic laparoscopy
• Diagnostic laparoscopy is regarded by many authorities as the standard for the diagnosis of acute PID.
• Criteria required for the diagnosis include abnormal erythema and edema of the fallopian tubes and sticky exudate on tubal surfaces and from fimbriated ends.10
TREATMENT
• Once the diagnosis of PID is made, 2010 CDC guidelines favor hospitalization under the following circumstances:
• A surgical emergency, such as ectopic pregnancy or acute appendicitis, cannot be adequately excluded
• A tubo-ovarian abscess is present
• Pregnancy
• Failure to respond clinically to oral antimicrobial therapy
• Severe illness, nausea and vomiting, or high fever
• Inability to follow or tolerate an outpatient oral regimen7
Medications
Antibiotic therapy is the cornerstone of treatment for acute PID. Empirical, broad-spectrum antimicrobial therapy targeting N. gonorrhoeae, C. trachomatis, enteric Gram-negative facultative bacteria (including Escherichia coli), and certain anaerobic bacteria is recommended.
• Inpatient regimens. Parenteral therapy can be discontinued as soon as 24 hours after the patient has improved clinically. Regimens recommended by the 2010 CDC guidelines are as follows:
• Doxycycline 100 mg IV (or PO) q12h, plus cefoxitin 2 g IV q6h (or cefotetan 2 g IV q12h), followed by doxycycline 100 mg PO bid for a total of 14 days.
• Clindamycin 900 mg IV q8h, plus gentamicin 2.0 mg per kg IV, followed by 1.5 mg per kg IV q8h, followed by either doxycycline 100 mg PO bid, or clindamycin 450 mg PO four times daily to complete 14 days of total therapy.7
• Outpatient regimens. Suggested regimens are as follows:
• Cefoxitin 2 g IM plus probenecid 1 g PO concurrently, or ceftriaxone 250 mg IM, plus doxycycline 100 mg PO bid for 14 days with or without metronidazole 500 mg PO bid for 14 days.
• Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime), plus doxycycline 100 mg PO bid for 14 days with or without metronidazole 500 mg PO bid for 14 days.7
Surgery
• Surgical treatment has a limited role in the management of PID.
• Possible indications include the confirmation of the diagnosis in a patient failing to respond to therapy, excision of chronically infected pelvic organs, and draining of pelvic abscesses.
Nonoperative
• General supportive measures, such as bed rest, sexual abstinence until cure is achieved, hydration, and provision of antipyretics and appropriate analgesia, are recommended in the management of PID.
• Although there is no evidence that IUDs have to be removed in women diagnosed with acute PID, if the IUD is not removed CDC guidelines mandate that close clinical follow-up be provided.
Follow-Up
• Patients should be seen within 3 days after initiation of therapy.
• Patients who fail to respond require careful reevaluation of both the diagnosis and therapy.
• Male sex partners who have had contact with the patient during the preceding 60 days should be evaluated and provided empiric treatment for Chlamydia and gonorrhea. If more than 60 days have elapsed since the patient’s last sexual intercourse, the patient’s most recent sexual partner should be treated.
• Patients should be provided counseling regarding safe sexual behavior, the use of condoms as a means for preventing the transmission of STDs, and the advisability of HIV testing.
• Periodic screening for Chlamydia is recommended in sexually active women at risk for this infection, such as unmarried women 25 years of age or younger.16
Complications
• Tubal factor infertility is seen in 8% to 12% of patients after one episode of PID, 20% to 25% after two episodes, and 40% to 50% after three episodes or more.
• Chronic pelvic pain has been reported in 15% to 20% of patients after PID.
• The risk of ectopic pregnancy is increased 3- to 10-fold in a patient with a history of PID.6,8
REFERENCES
1. Sutton MY, Sternberg M, Zaidi A, et al. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sex Transm Dis 2005;32:778–84.
2. Gradison M. Pelvic inflammatory disease. Am Fam Physician 2012;85:791–796.
3. Soper DE. Pelvic inflammatory disease. Obstet Gynecol 2010;116:419–428.
4. Sweet RL. Treatment of acute pelvic inflammatory disease. Infect Dis Obstet Gynecol 2011;2011:561909. doi: 10.1155/2011/561909.
5. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect 2006;82:452–457.
6. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis 2013;40:117–122.
7. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR 2010;59(No. RR-12):63–69.
8. Banikarim C, Chacko MR. Pelvic inflammatory disease in adolescents. Semin Pediatr Infect Dis 2005;16:175–180.
9. Quan M. Pelvic inflammatory disease: diagnosis and management. J Am Board Fam Pract 1994;7:110.
10. Jaiyeoba O, Soper DE. A practical approach to the diagnosis of pelvic inflammatory disease. Infect Dis Obstet Gynecol 2011;2010:753037. doi: 10.1155/2011/753037.
11. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol 2008;111:1479–1485.
12. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin N Am 2013;27:793–809.
13. Cicchiello LA, Hampe UM, Scoutt LM. Ultrasound evaluation of gynecologic causes of pelvic pain. Obstet Gynecol Clin North Am 2011;38:85–114.
14. Li W, Zhang Y, Cui Y, et al. Pelvic inflammatory disease: evaluation of diagnostic accuracy with conventional MR with added diffusion-weighted imaging. Abdom Imaging 2013;38:193–200.
15. Vandermeer FQ, Wong-You-Cheong JJ. Imaging of acute pelvic pain. Clin Obstet Gynecol 2009;52:2–20.
16. Gottlieb SL, Xu F, Brunham RC. Screening and treating chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized controlled trials. Sex Trans Dis 2011;40:97–102.
|
GENERAL PRINCIPLES
Definition
Menopause is defined as the failure of ovarian follicle development in the presence of adequate gonadotropin stimulation, resulting in the cessation of spontaneous menstrual periods. A woman is considered to be postmenopausal after 12 months of amenorrhea without another physiologic or pathological cause.
Epidemiology
The average age for menopause is 51 years.1 Factors that can lower the age of menopause include smoking, hysterectomy, oophorectomy, genetic disorders, autoimmune disorders, high altitude, and a history of chemotherapy or radiation.
Classification
Menopause may be secondary to surgical intervention or drug effect, particularly chemotherapeutic agents. Evidence of menopause before 40 years of age is considered premature ovarian failure and usually necessitates a workup.
Pathophysiology
Vasomotor symptoms are not fully understood but may relate to estrogen withdrawal and a narrowed thermoregulatory zone, resulting in increased sensitivity to temperature change. Symptoms are often worse in obese women, possibly due to the insulating effects or endocrine effects of adipose tissue. Vasomotor symptoms are also associated with depression, anxiety, smoking, and low socioeconomic status.1 The vaginal epithelium is estrogen-dependent, and declining estrogen levels lead to thinning of the vaginal mucosa, loss of rugae, and narrowing and shortening of the vagina. There is also a loss of subcutaneous fat in the labia majora. Vaginal pH increases, altering vaginal flora, and vaginal secretions decrease. These changes may result in infection, fissures or tears, fusion of labia minora, and shrinking of the clitoris and urethra. Decreased libido may be secondary to dyspareunia, stress, depression, or hormonal changes. Estrogen deficiency leads to decreased osteoblastic activity with increased osteoclastic activity, resulting in osteoporosis and risk for fractures.
DIAGNOSIS
Clinical Presentation
Perimenopause, or the menopausal transition, often begins as early as 6 years before the last period.1 During this time, women may experience signs or symptoms such as irregular periods, mood changes, insomnia, weight gain, bloating, vaginal dryness, decreased libido, headaches, or vasomotor instability (hot flashes). Hot flashes, which affect 80% of perimenopausal women, are described as intense warmth and profuse sweating, which may be accompanied by palpitations and redness of the skin. They last from seconds to minutes, rarely up to 1 hour, and may occur as often as 20 times per day. As ovarian response to gonadotropins declines in the postmenopausal years, associated symptoms decrease, also. After menopause, fibroids, endometriosis, and adenomyosis become less symptomatic. Prolapse of genitourinary organs may occur as loss of pelvic muscle tone occurs. Atrophic vaginitis may lead to insertional dyspareunia. Atrophic cystitis may mimic a urinary tract infection (UTI), and is also a risk factor for UTIs.
History
The history should focus on gynecologic and cardiovascular history, family history of breast or uterine cancer, and risk factors for osteoporosis and coronary artery disease. Note the frequency and severity of menopausal symptoms and their effect on the patient’s overall function.
Physical Examination
The physical examination should be complete, including vital signs, thyroid, cardiovascular, breast, and pelvic examination. Pelvic examination at first reveals reddened vaginal epithelium as the skin thins and capillaries are more visible. After time, the number of capillaries decreases and the vaginal epithelium becomes pale. Rugation of the vaginal mucosa decreases, the uterus and ovaries diminish in size, and loss of pelvic tone may lead to prolapse. A palpable ovary on bimanual examination in a postmenopausal woman is abnormal and warrants a full evaluation. Pap smear should be completed if not up-to-date.
Laboratory Studies
A persistently elevated follicle-stimulating hormone (FSH) level confirms the diagnosis of menopause. Eventually FSH will increase 10- to 20-fold, while luteinizing hormone (LH) will increase to three times that of premenopausal levels. Conversely, estradiol and inhibin levels decline. Estradiol, inhibin, and LH values are not necessary to diagnose menopause. If the diagnosis is uncertain, repeat measurement of FSH and LH every 2 to 3 months may be helpful. Laboratory testing may include thyroid function tests or other tests as indicated by history and physical examination. Urine culture should be done for women complaining of UTI symptoms, even if they may be related to atrophic cystitis.
Imaging
Imaging is not necessary to diagnose menopause. Pelvic ultrasound to assess for endometrial hyperplasia is recommended in postmenopausal uterine bleeding. Bone densitometry testing should be done starting at 65 years of age or earlier for those with risk factors. Bone loss accelerates in the late perimenopausal years and continues after menopause. Mammograms are currently recommended for all women ages 50 to 74 and may be indicated for certain women at younger ages.
Surgical Diagnostic Procedures
Endometrial biopsy to rule out endometrial cancer should be performed on women with abnormal uterine bleeding and an endometrial stripe measuring more than 5 mm by ultrasound.
Differential Diagnosis
The differential diagnosis of vasomotor instability includes alcohol withdrawal, anxiety disorders, carcinoid tumor, epilepsy, insulin reaction, pheochromocytoma, thyrotoxicosis, and drug effects.
TREATMENT
Treatment of menopausal symptoms should be tailored to the severity of symptoms. Mild symptoms may be treated with behavioral modification, whereas more severe symptoms may require pharmacologic therapy. There is a significant placebo effect in the treatment of hot flashes, with up to 25% reduction in the number of hot flashes with placebo in controlled trials.1
Behavioral
Nonpharmacologic treatments for hot flashes include fans; cool drinks; lower ambient temperature; loose or layered clothing; and avoidance of alcohol, caffeine, and spicy foods. Risk of osteoporosis may be reduced by weight-bearing exercise and smoking cessation.
Medications
Estrogen has been used in the treatment of perimenopausal symptoms for more than 50 years. Selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), gabapentin, and clonidine can be useful alternatives to hormonal therapies for treatment of vasomotor symptoms. Paroxetine is the only nonhormonal treatment approved by the FDA for treatment of vasomotor symptoms. The FDA has approved ospemifene for treating moderate-to-severe dyspareunia in postmenopausal women. Estrogens, selective estrogen receptor modulators (SERMs), and bisphosphonates are approved for the prevention and treatment of osteoporosis.
• Hormone replacement therapy (HRT) is the most effective therapy for vasomotor symptoms of menopause. HRT may also improve mood symptoms, fatigue, incontinence, and vaginal dryness. Low-dose estrogen (0.3 to 0.45 mg per day conjugated estrogen, 0.5 mg per day micronized estradiol, 5 mcg per day ethinyl estradiol, or 0.025 to 0.0375 mg per week transdermal estradiol) or ultra-low-dose estrogen (0.25 mg per day micronized estradiol or 0.014 mg per week transdermal estradiol) regimens have a better side effect profile and may be effective. HRT may be administered orally or transdermally in the forms of patches, gels, or sprays. Contraindications to estrogen therapy include undiagnosed vaginal bleeding, liver disease, pregnancy, venous thromboembolism (VTE), and personal history of breast cancer. Well-differentiated early endometrial cancer after complete treatment is no longer an absolute contraindication.
• Risks of HRT: The Women’s Health Initiative (WHI) study, a large randomized controlled trial (RCT) of healthy women 50 to 77 years old, demonstrated that after an average of 5 years of combined HRT, women had slightly increased risk of coronary artery disease, stroke, VTE, and breast cancer and a decreased risk of colon cancer and fractures.2 For women receiving estrogen without progestin, there was an increased risk of VTE but not of cardiovascular disease or breast cancer.3 It should be noted that these women were already past the menopausal transition, so it is difficult to extrapolate these data to general populations. Later analysis of the WHI data in women 55 to 60 years of age and within 10 years of menopause suggests a possible cardioprotective effect of HRT for these younger women. However, follow-up of these women at 13 years confirmed that the risks of combined HRT for primary prevention outweigh the benefits.4 A Cochrane review of HRT completed in 2012 similarly showed that HRT should not be used for primary prevention because the risks outweigh the benefits. Different forms of HRT may have different risk profiles based on observational studies, and additional randomized trials are needed to further investigate this. Yearly mammograms are necessary for all women on HRT. An on-going discussion should be held at each healthcare maintenance visit regarding symptoms and treatment, with the goal of using the lowest possible dose of HRT for the shortest amount of time possible for each individual patient. Discontinuation may lead to return of vasomotor symptoms in up to half of women, regardless of age and duration of use. There are insufficient data to recommend tapering the dose versus abrupt cessation.
• Estrogen/progestin combination therapy is necessary in patients with an intact uterus or with endometriosis remaining after hysterectomy to avoid endometrial hyperplasia and development of endometrial cancer. Some studies suggest that addition of progestin to estrogen replacement leads to greater improvement of vasomotor symptoms. The progestin in combination therapy may be dosed in either a continuous or a cyclic manner.
• Continuous progestin dosing causes breakthrough bleeding in half of women in the first 6 months of therapy. Endometrial biopsy is indicated for breakthrough bleeding beyond this time frame. The progestin dose may be doubled if no pathology is found on biopsy.
• Cyclic progestin dosing causes less breakthrough bleeding but may worsen migraine headaches. Nonsmoking perimenopausal women may use low-dose oral contraceptive pills for regulation of menses, relief of vasomotor symptoms, and contraception.
• Progestin-only therapy has some evidence of relieving vasomotor symptoms. However, there are limited data on the safety of progestin alone. Furthermore, the rate of breast cancer was higher in the combined arm than in the estrogen-only arm of the WHI, indicating a possible correlation between progestin therapy and breast cancer risk.
• Vaginal estrogen in the form of cream, ring, or tablet may reverse vaginal atrophy if dosed daily for 1 to 2 weeks. This benefit is maintained with two to three treatments per week or a decreased daily dose. There is theoretical concern for endometrial hyperplasia or cancer in long-term vaginal estrogen therapy. However, a Cochrane meta-analysis showed no increase in either of these outcomes, so addition of progestin is not necessary for patients using vaginal estrogen.1 Vaginal estrogen at low doses may be used indefinitely. The 3-month estradiol-releasing vaginal ring is often easier to use than daily tablets or creams.
• SERMs such as tamoxifen and raloxifene have not been shown to be beneficial in treating vasomotor or vaginal symptoms. Ospemifene (60 mg per day) is a novel SERM that has been shown to improve vaginal atrophy without stimulating the endometrium, and was recently approved by the FDA for treatment of moderate-to-severe dyspaurenia in postmenopausal women. Side effects include hot flashes, vaginal discharge, muscle spasms, and excessive sweating.
• Estrogen/SERM combination therapy may be an alternative to estrogen plus progestin. A combination of conjugated estrogen and bazedoxifene (Duavee) has been approved by the FDA recently for treatment of vasomotor symptoms and to prevent osteoporosis in postmenopausal women with a uterus. This medication significantly reduces the number of vasomotor symptoms and increases bone mineral density compared to placebo.5
• Plant-derived phytoestrogens found in soy, wheat, cereals, nuts, and apples are converted to estrogens in the gut. These estrogens may have agonist and/or antagonist activity when bound to estrogen receptors. Controlled trials have not shown these to be efficacious for treatment of vasomotor symptoms.
• Testosterone therapy has not been shown to decrease vasomotor symptoms, and has adverse effects of acne, hirsutism, and dyslipidemia. A Cochrane review did show that addition of testosterone to HRT improves sexual function in postmenopausal women.6
• SSRIs and SNRIs such as paroxetine, citalopram, fluoxetine, and venlafaxine may reduce hot flashes. Side effects may include dry mouth, dizziness, nausea, constipation, sweating, and sexual dysfunction, but these often resolve with time or dose adjustment. Only paroxetine (7.5 mg per day) is FDA-approved for treatment of vasomotor symptoms.
• Gabapentin (900 mg per day) is associated with a 45% decrease in frequency and 54% decrease in severity of hot flashes.7 Side effects include dizziness, somnolence, and peripheral edema.
• Clonidine (0.1 mg per day) may reduce hot flashes, although data are limited. Possible side effects include insomnia, dry mouth, and drowsiness.
• Vitamin E therapy (800 U per day) has had anecdotal reports of improvement in hot flashes. However, no significant benefit was noted over placebo during controlled trials.
• Water-based or silicone-based lubricants may help symptoms of vaginal dryness.
• Data do not support the use of compounded bio-identical hormones; synthetic steroids; alternative therapies such as acupuncture and reflexology; or herbal remedies such as black cohosh, evening primrose, or red clover extract. Black cohosh in particular should not be used for more than 6 months due to concern for liver toxicity.
Follow-Up
Follow-up in 3 to 6 months after initiation of therapy for menopausal symptoms is recommended to determine the adequacy of the regimen and review side effects. Once an appropriate regimen has been established, the patient should be evaluated yearly.
REFERENCES
1. ACOG practice bulletin 141: management of menopausal symptoms. Obstet Gynecol 2014;123:202–216.
2. Rossouw J, Anderson G, Prentice R, et al.; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333.
3. Anderson G, Limacher M, Assaf A, et al.; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712.
4. Manson J, Chlebowski R, Stefanick M, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013;310:1353–1368.
5. Tella SH, Gallagher JC. Bazedoxifene + conjugated estrogens in HT for the prevention of osteoporosis and treatment of vasomotor symptoms associated with the menopause. Expert Opin Pharmacother 2013;14:2407–2420.
6. Somboonporn W, Bell R, Davis S. Testosterone for peri and postmenopausal women. Cochrane Database Syst Rev 2005;(4):CD004509. doi: 10.1002/14651858.
7. Guttuso T Jr, Kurlan R, McDermott M, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–345.
| Benign Breast Conditions and Disease |
GENERAL PRINCIPLES
Definition
Benign breast conditions encompass a multitude of conditions united only in that cancer and precancerous lesions are excluded. Many women present to their physician for benign conditions of the breast that they perceive to be abnormal. Common complaints include pain, hypertrophy, breast lumps, breast infections, and nipple discharge. The health care provider must differentiate benign from malignant disease, reassure patients with benign conditions, manage common symptoms and conditions, and seek consultation when necessary. The provider must recognize the emotional distress common during this process and provide timely and effective communication.
Anatomy and Breast Development
The adult breast is a tear-shaped milk-producing gland supported and attached to the chest wall by the Cooper suspensory ligaments. The adult breast is located between the second and sixth ribs in the vertical axis, between the sternal edge and the midaxillary line in the horizontal axis, and extends into the axilla. Each breast is composed of 15 to 20 lobes, each composed of multiple lobules. Glandular milk-producing lobules drain through a series of branching ducts to the nipple, and are supported by fibrous tissue, or stroma. Typically, there are 6 to 10 pinhole openings on the areola, each draining a duct that leads to a single lobe. Because more lobes are present in the outer quadrants, especially the upper outer quadrants, many breast conditions (including breast cancer) occur more frequently in these regions.1
Breast development and change may be seen as a process of dynamic change beginning with embryonic development and continuing through the postmenopausal years. Newborns commonly have hypertrophied breast tissue caused by stimulation from maternal estrogen and progesterone. In most cases spontaneous regression occurs. Prepubertal children may develop unilateral or bilateral soft mobile subareolar nodules of uniform consistency that usually resolve spontaneously within a few months. Biopsy should be avoided as it may impair pubertal breast development. In girls, puberty marks the normal onset of glandular proliferation within the breast. For most girls, breast bud development (thelarche) is the first sign of puberty (typically between ages 10 and 12), while full breast development is usually the last sign. Thelarche is considered “premature” if it occurs earlier than age 8. Premature thelarche without other signs of pubertal development or accelerated growth is usually benign. No treatment is needed if medical evaluation excludes true precocious puberty, estrogen-producing tumors, ovarian cysts, or exogenous estrogen exposure. Other signs of puberty generally begin within 6 months of breast development and are completed within 4 years. Breast development may begin on one side and be asymmetrical. If there is a discrepancy in size, the left breast is usually larger. Breast development is considered “delayed” if stage 1 persists beyond 13.5 years; stage 2 persists more than 1 year; stage 3 greater than 2.2 years; or stage 4 more than 6 to 8 years.1
The normal breast changes in size and texture throughout the menstrual cycle as well. During the premenstrual phase, acinar cells, or the cells of the terminal duct-lobule unit, increase in number and size, the ductal lumens widen, and breast size and turgor increase. These changes reverse in the postmenstrual phase.1
Gynecomastia, or the proliferation of glandular breast tissue in a male, is common in the middle phases of pubertal development and in adulthood. In puberty, this may be attributed to serum estradiol levels rising to adult levels before serum testosterone levels. Although it can be psychologically disturbing, workup is indicated only if there is rapid progression, onset before puberty, or association with true precocious puberty. More than 90% of affected boys experience regression within 3 years.2
Classification of Conditions
Nipple Anomalies
The most common anomaly is polythelia, or, accessory nipple. Ectopic nipple tissue may occur at any point in the embryonic breast line, from the groin to the axilla. In many instances, an accessory nipple may be misdiagnosed as a nevus or dermatofibroma. Other nipple findings include discharge, Paget disease, and painful nipples.
Breast Infections
Infectious disease of the breast may include mastitis, abscess, or cellulitis. In evaluating and treating infections of the breast, it is important to determine whether the woman is lactating.
Structural/Functional Anomalies
These changes encompass many common complaints, including palpable masses as well as cyclical and noncyclical breast pain.
ASSESSMENT OF AN INDIVIDUAL WITH BREAST COMPLAINTS
History
In taking the history of the breast complaint, it is important that many areas of relevant history are obtained. Questions should focus on areas such as breast lump characteristics, diet and medications, family history, past medical and surgical history, social history, gynecological history, and context in which the breast lump was discovered.
In gathering history of breast lump characteristics, questions should be focused on changes in size of lump over time and in relation to menstrual cycle, how long the mass has been present, and if any pain, swelling, erythema, or discharge has been present. Past and current medications along with whether or not the patient has been on hormone therapy should be documented also. Important medical and surgical history should include questions about any personal history of breast cancer, previous lumps or biopsies, recent breast trauma, and any past or current radiation or chemotherapy. Family history should include information on any family member with a history of breast or ovarian cancer. Along with past medical, surgical, and family history, a social history should be obtained and include tobacco use, illicit substance use, and alcohol use. Finally, a gynecological history should be obtained and include age at first childbearing, age at menarche, age at menopause if applicable, current lactation status, history of breastfeeding, and number of children.3–5
Examination of the Breast
• Inspection is first followed by palpation. The examination should be performed in a well-lit room, and privacy is facilitated by draping parts of the body not being examined. Inspection occurs with the patient seated, arms at side; seated with hands on hips; seated with arms above the head; and supine with one arm raised at a time. Changes in size, shape, symmetry, or texture are noted. Palpation is performed with the patient supine, arms flexed at a 90-degree angle at the sides utilizing the “triple touch” technique of palpation of superficial, intermediate, and deep tissue planes. Palpation includes supraclavicular, infraclavicular, and axillary nodes. Compression may identify a mass and/or elicit a discharge. Nipples should be examined for deviation, retraction, skin changes, or discharge.6
Laboratory Evaluation
Currently, genetic screening is not part of the routine evaluation of individuals with breast-related complaints.
Diagnostic Tests
• Imaging. Mammography is discussed elsewhere in this manual. When mammography is indicated, a “diagnostic” rather than a “screening” mammogram is obtained to evaluate women with breast complaints. Important diagnostic information may be obtained regarding a known or undetected mass. However, a negative mammogram should never preclude biopsy of an appropriate palpable lesion. Since mammography is unable to visualize lesions well in younger women, ultrasonography may be preferable in women under 30 years of age to differentiate whether a mass is solid or cystic and as an adjunct to aspiration or biopsy. Magnetic resonance imaging is utilized in patients with silicone breast implants, women with a family history of breast cancer or known genetic susceptibility, and special circumstances. These circumstances include patients who have had breast conserving surgery, have known carcinoma needing further evaluation, have axillary metastasis and unknown primary tumor, have extensive postoperative scarring, or have extremely dense stroma.6
• Aspiration. A cystic lesion or lesion of uncertain nature may be aspirated both diagnostically and therapeutically.6
• Fine-needle aspiration (FNA). FNA involves cytologic aspirate of a mass using a 22- to 25-gauge needle usually with ultrasound or stereotactic guidance. Specimen must have adequate number of epithelial cells for interpretation (sensitivity 98% to 99% for malignancy).6
• Fine-needle aspiration and biopsy (FNAB). Indications for biopsy include any suspicious lesion; bloody nipple discharge or bloody fluid following cyst aspiration; persistent mass; suspicious skin changes; inflammatory changes unresponsive to antibiotic; suspicious axillary nodes; or suspicious microcalcifications on mammography. A 14- to 18-gauge needle is used to obtain six scores of the mass for histology.6
• Triple test. The “triple test” combines physical examination, mammography, and FNAB. The test utilizes a Triple Test Score. The three-point scale is used for each component of the test. A score of 1 is benign, 2 is suspicious, and 3 is malignant. A Triple Test Score of 3 to 4 is consistent with a benign lesion, a score of 5 indicates that further information should be obtained such as an excisional biopsy, and a score of greater than 6 indicates possible malignancy is present.6
• Excisional biopsy. The excisional biopsy is the gold standard for evaluating breast masses. The entire lesion is removed usually in an operating room. The biopsy can be both diagnostic and therapeutic if negative margins are present. Due to the increase in FNAB, excisional biopsies have declined.6
Pathologic Findings
Benign breast lesions diagnosed by the above methods may be subdivided based on the degree of risk they confer for the future development of malignancy. Such categorization is usually determined by proliferation and atypia. Lesions associated with an increased risk of developing breast cancer include any proliferative lesion, common benign lesions in patients older than 50 (such as cyst, adenosis, mammary duct ectasia fibrosis, metaplasia, fibroadenoma), mild/moderate or florid hyperplasia without atypia and simple papilloma. This risk is likely increased in patients with a strong family history of breast cancer. Findings with a small increase in relative risk include ductal hyperplasia without atypia, sclerosing adenosis, diffuse papillomas, complex fibroadenomas, and radial scars. Lesions conferring moderately increased risk include atypical ductal hyperplasia and atypical lobular hyperplasia. Both of the aforementioned lesions also confer an increased risk for development of breast cancer in the contralateral breast.
Emotional well-being of the patient. The evaluation of a breast complaint is extremely stressful for many women. Most patients assume that their sign or symptom indicates cancer. The provider should anticipate the emotional responses typical in patients and family members. Timely assessment, diagnostic evaluation, and consultation when necessary should be provided. It may be useful to inquire how best to assist with the period of uncertainty and how the patient would like results conveyed to her. Realistic estimates of the likely time involved in diagnosis are beneficial. Adequate time should be made available to address questions and additional methods of contact (office visits, telephone calls, or e-mail) should be offered.4
BREAST PAIN
Mastalgia is an extremely common complaint among women accounting for 66% of physician visits for breast complaints, which may interfere significantly with quality of life. Pain without an associated mass is unlikely to be the presenting symptom of breast cancer, although evaluation may lead to the coincidental diagnosis of cancer. Mastalgia may be classed as cyclical or noncyclical, and may be acute or chronic.
• Epidemiology. Mastalgia is a more common complaint in premenopausal women than in postmenopausal women.
• Classification/etiology. Breast pain may be classified as cyclical (2/3) or noncyclical (1/3). Cyclical breast pain commonly occurs as a result of estrogen stimulation of ductal elements, and progesterone stimulation of the stromal such as during the normal menstrual cycle. Noncyclical causes of pain may include extramammary or chest wall pain, neuropathic pain, trauma, fat necrosis, infection, painful breast mass or cyst, and a multitude of other causes.
• History. Pertinent historical details include alleviating or aggravating factors, quality, radiation, severity, location, and laterality. It is important to illicit timing with regard to menstrual cycle, association with oral contraceptive pills or hormone replacement use, recent birth, pregnancy, loss of pregnancy, or termination. History of trauma, heavy muscular exertion, and constitutional symptoms should be sought.
• Physical examination. Physical examination should be used to evaluate for mass or nipple discharge; to localize areas of tenderness; and to assess for lymphadenopathy and changes in symmetry, contour, and overlying skin.
• Laboratory studies and imaging. Standard laboratory testing may be sought to assess for infection. Mammography or ultrasound may be useful in assessing for masses.3,5
Noncyclical Breast Pain
• Trauma. Trauma may produce a hematoma or rupture of a cyst and may also lead to fat necrosis. The patient will typically complain of pain and tenderness following an injury. Mild swelling and discoloration may be present. Unless a coagulopathy is suspected, no diagnostic tests are indicated. Fat necrosis is usually caused by preceding trauma and may be more likely if there is a history of fibrocystic breast disease. Physical examination reveals localized pain, swelling, and erythema. Evaluation should be performed to exclude malignancy if symptoms persist for more than a week, although fat necrosis commonly results in a residual calcified mass.
• Ductal ectasia. Distention of subareolar ducts due to inflammation may lead to pain of the breast. Typically fever with acute local pain and tenderness is associated. The degree of pain is correlated to the degree of inflammation.
• Hormone replacement therapy. Up to one-third of women receiving postmenopausal hormone therapy may experience breast pain. This typically will resolve over time.
• Large pendulous breasts. Large breasts may stretch Cooper’s ligaments, which can cause pain. Typically the breasts will be pendulous in nature, and other myalgias such as the neck, back, or shoulder may be present.
• Lactation-related pain. Breast engorgement is a common cause of breast pain in new mothers. Breast engorgement usually occurs on the second or third postpartum day. Lactating women may also develop pain secondary to a galactocele or milk-filled cyst. These often resolve spontaneously.
• Mastitis. Both mastitis and breast abscesses almost always occur in lactating women or in women with a history of a bite or penetrating trauma. Mastitis commonly presents 1 week or more after delivery. Moderate to severe pain, tenderness, erythema, swelling, and warmth are usually localized to one breast, often to one quadrant or lobule. Axillary adenopathy may be present and there may be purulent drainage. The patient may be febrile and appear toxic. History and physical examination are diagnostic. Leukocytosis is common. Breast milk cultures are not useful, and Staphylococcus aureus is typically causative in breastfeeding women. Treatment for mild infection includes anti-inflammatory agents and cold compresses along with 10 to 14 days on oral antibiotics deemed safe for a nursing infant. Dicloxacillin 500 mg qid; cephalexin 500 mg qid; or clindamycin 300 mg qid for penicillin-allergic patients are recommended if a low suspicion for methicillin-resistant S. aureus (MRSA) is present. If a high suspicion for MRSA is present, clindamycin 300 mg qid, trimethoprim–sulfamethoxazole one to two tabs bid, or linezolid 600 mg bid is recommended. Patients should be reassessed in 48 to 72 hours. Breastfeeding should be continued on the affected breast to encourage drainage (the infant is not at risk for developing infection). Breast pumping is also appropriate.
• Abscess. Pitting edema over an area of inflammation and fluctuation is suggestive of abscess development. For patients with infections unresponsive to conservative management, severe infection, abscess, or deep infection, the wound should be drained and cultured. Breastfeeding should be continued and oral antibiotics should be started. Antibiotic regimens are the same as for mastitis listed above. If hemodynamic instability is present, hospitalization and initiation of vancomycin should be started. Nonpuerperal abscesses are usually caused by anaerobic bacteria if subareolar and by staphylococci in other locations. Nonpuerperal abscesses are treated with clindamycin or metronidazole. If the clinical setting is atypical, the woman is not breastfeeding, or she does not improve with antibiotics, a biopsy of indurated areas to exclude an underlying cancer should be considered. Presence of a periareolar inflammatory mass, breast abscess in a nonlactating woman, or a mammary duct fistula should raise suspicion of periductal mastitis.5–7
Cyclical Breast Pain
• Etiology. Most cyclical breast pain is associated with the menstrual cycle. Pain is usually worse in the luteal phase and abates following menstruation. Most women report some degree of cyclical breast pain at some point in their lives; 21% experience severe pain that interferes with function. Pain is typically bilateral and diffuse. Cyclical breast pain is not always associated with premenstrual syndrome (PMS), but 60% of women with PMS report breast pain as the predominant symptom. There is a high likelihood of spontaneous resolution of cyclical breast pain.
• Treatment. Watchful waiting and reassurance may be acceptable in up to 85% of patients. Breast support and analgesia with acetaminophen and anti-inflammatories may be beneficial. There is no evidence of benefit from dietary change. Progesterone and diuretics have also not been proved to be effective. Danazol has significant adverse effects (voice change, hirsutism, weight gain, acne), but 200 mg daily in the luteal phase (day 14 to 28) may be effective and minimize the total dose.7 Tamoxifen 10 mg daily is efficacious as a continuous dose only during the luteal phase, but side effects limit its long-term use. Bromocriptine- and gonadotropin-releasing hormone agonists have also been successfully used for severe pain but are associated with a number of side effects. Additional therapies may include:
• Evening primrose oil 500 mg, two tablets tid, has been demonstrated efficacious in a randomized controlled clinical trial with no apparent adverse effects.
• Lowering the dose of estrogens in the treatment of postmenopausal women may be helpful, and the addition of an androgen to hormone replacement therapy may alleviate symptoms as well.
• The use of oral contraceptives has not been well studied, but low-dose estrogen and 19-norprogesterone may be effective in relieving symptoms.3,5
Breast Mass
Benign breast masses are most commonly fibroadenomas or cysts, but must be differentiated from malignant disease. Benign breast masses will often change with the menstrual cycle, while worrisome masses are persistent throughout. Greater than 90% of palpable breast masses in women between 20 and 55 are benign. Masses may be discrete or poorly defined, but differ from the surrounding breast tissue and the corresponding area in the contralateral breast. Cancer should be excluded in a woman who presents with a solid mass. A woman with a clinically suspicious lesion should undergo mammography and/or ultrasound, and biopsy. Characteristics of a mass that are concerning for malignancy include single lesion, hard, fixed/immobile, irregular border, and size greater than 2 cm. Any asymmetry, skin dimpling, nipple discharge, and lymphadenopathy must also be assessed.3,7
• Breast cysts. Cysts may be solitary or multiple, and may be difficult to differentiate from solid masses on physical examination. Cystic disease peaks women 35 to 50 years of age. Ultrasound may be used to differentiate solid versus cystic masses and aspiration may be both diagnostic and therapeutic. Cysts should be surgically biopsied if they contain bloody fluid, fail to resolve completely after drainage, or recur after 4 to 6 weeks. It is not necessary to send aspirated fluid for cytologic examination. Nonpalpable cysts identified during routine mammography do not require further evaluation or treatment.
• Fibrocystic breast changes. Fibrocystic changes are the most common benign condition of the breast. Most experts consider such changes to be part of the natural history of the breast as histologic changes consistent with this diagnosis may be found in the majority of asymptomatic women. Changes are most common in women 35 to 45 years old and are rare in postmenopausal women. No treatment is necessary unless the woman is symptomatic (for example, from an enlarging cyst) or if physical findings are worrisome for possible cancer.
• Anatomy. Fibrocystic changes consist of an increased number of cysts or fibrous tissue in an otherwise normal breast.
• Epidemiology. Fibrocystic change may be divided into three subgroups with predominant histologic characteristics and age distributions. Hyperplasia commonly occurs in women in their 20s and presents as stromal proliferation and pain in the upper and outer quadrants. Adenosis is caused by proliferation of glandular cells and presents commonly as multiple 2- to 10-mm breast nodules in women in their 30s. Cystic disease occurs more commonly in women older than 40 with the painful enlargement of multiple or solitary cysts.
• History. When symptoms are present, the most common symptom is cyclical pain (mastalgia). The pain is generally bilateral, located in the upper outer quadrants, begins a few days prior to menstruation, diminishes with the onset of menses, and may be associated with an increase in breast size. Family history is common.
• Physical examination. Cysts are smooth, regular, rubbery, and easily movable lumps or areas of local tenderness without a discrete mass. Cysts can range in size from 1 mm to many centimeters. Compression causes tenderness. Larger cysts are more common as women age. To assess for possible menstrual changes, it may be helpful to repeat the examination with the patient at another point in her cycle. It may be difficult to discern a cyst from a solid mass on physical examination. Pale green to brown nipple discharge may be noted in cystic disease.
• Diagnostic evaluation. Although fibrocystic symptoms typically differ from those associated with malignancy, if there is any doubt regarding the diagnosis or if a single mass is present, further evaluation for breast mass is needed. There is no increased risk of cancer in women with fibrocystic changes in a woman younger than 50 years unless proliferative or hyperplastic lesions with atypical epithelial cells are present on biopsy.
• Management. Most women do not require treatment. Treatment, if necessary, is focused on the predominant symptom or sign such as pain or a mass. A well-padded support bra and loose light clothing may relieve discomfort and weight reduction is recommended in women with a body mass index greater than 30 mm per kg. Calcium may be beneficial, but many other previously recommended therapies (dietary restriction of caffeine and methylxanthines in chocolate, tea, coffee, soda, and theophylline; use of vitamins, including A, E, and thiamine; and use of diuretics) have not proven efficacious in randomized controlled clinical trials. Additional management may include the following:
![]() Low-estrogen/high-progesterone oral contraceptives may be used, but the patient may not notice significant change until 1 to 2 years of use.
Low-estrogen/high-progesterone oral contraceptives may be used, but the patient may not notice significant change until 1 to 2 years of use.
![]() Progesterone, such as medroxyprogesterone 5 to 10 mg daily for 10 days before menses, may be given for a trial of 4 to 6 months. Side effects may include weight gain, depression, breakthrough bleeding, and lipid alterations.
Progesterone, such as medroxyprogesterone 5 to 10 mg daily for 10 days before menses, may be given for a trial of 4 to 6 months. Side effects may include weight gain, depression, breakthrough bleeding, and lipid alterations.
![]() If thyroid-stimulating hormone (TSH) is elevated even when other thyroid hormones are normal, a trial of thyroid replacement may be helpful.
If thyroid-stimulating hormone (TSH) is elevated even when other thyroid hormones are normal, a trial of thyroid replacement may be helpful.
![]() cis-Linoleic acid (evening primrose oil) at a dose of 1 g every 8 hours may be beneficial. However, the benefit may not be seen for 3 to 4 months.
cis-Linoleic acid (evening primrose oil) at a dose of 1 g every 8 hours may be beneficial. However, the benefit may not be seen for 3 to 4 months.
![]() Danazol is the only pharmacologic agent approved by the U.S. Food and Drug Administration for treatment of fibrocystic breast changes. Many women achieve the needed benefit, but significant side effects (hirsutism, amenorrhea, weight gain of 4 to 6 pounds, hot flashes, and acne) are common. Danazol is generally reserved for women with severe symptoms. Dose at 200 to 800 mg per day PO initially and then may decrease to 50 to 100 mg per day as maintenance once response is achieved. Some women benefit from 200 mg daily, given on days 14 to 28 of the menstrual cycle. Duration of treatment is usually limited to 3 to 6 months. Once danazol is discontinued, the treatment response may persist for months to years.
Danazol is the only pharmacologic agent approved by the U.S. Food and Drug Administration for treatment of fibrocystic breast changes. Many women achieve the needed benefit, but significant side effects (hirsutism, amenorrhea, weight gain of 4 to 6 pounds, hot flashes, and acne) are common. Danazol is generally reserved for women with severe symptoms. Dose at 200 to 800 mg per day PO initially and then may decrease to 50 to 100 mg per day as maintenance once response is achieved. Some women benefit from 200 mg daily, given on days 14 to 28 of the menstrual cycle. Duration of treatment is usually limited to 3 to 6 months. Once danazol is discontinued, the treatment response may persist for months to years.
![]() Tamoxifen, an antiestrogen, reduced breast pain in approximately 70% of patients within 3 to 6 months in several studies. In premenopausal women younger than 49 years, 20 mg per day was found to cause no increase in the incidence of deep venous thrombus, pulmonary embolism, stroke, transient ischemic attack (TIA), or endometrial cancer. Tamoxifen 10 mg per day may be effective when used only during the luteal phase (days 15 to 25), but its use should be restricted to fewer than 6 months. Side effects may include hot flashes, gastrointestinal (GI) symptoms, and vaginal discharge.
Tamoxifen, an antiestrogen, reduced breast pain in approximately 70% of patients within 3 to 6 months in several studies. In premenopausal women younger than 49 years, 20 mg per day was found to cause no increase in the incidence of deep venous thrombus, pulmonary embolism, stroke, transient ischemic attack (TIA), or endometrial cancer. Tamoxifen 10 mg per day may be effective when used only during the luteal phase (days 15 to 25), but its use should be restricted to fewer than 6 months. Side effects may include hot flashes, gastrointestinal (GI) symptoms, and vaginal discharge.
![]() Surgery (subcutaneous mastectomy; oophorectomy) should be considered only after medical management has failed for women with recalcitrant symptoms. Surgery may be useful for patients with one large dominant cyst.
Surgery (subcutaneous mastectomy; oophorectomy) should be considered only after medical management has failed for women with recalcitrant symptoms. Surgery may be useful for patients with one large dominant cyst.
![]() Bromocriptine- and luteinizing hormone–releasing agents have also been used but have significant side effects.3,5
Bromocriptine- and luteinizing hormone–releasing agents have also been used but have significant side effects.3,5
• Fibroadenoma. Fibroadenoma, the most common solid tumor, contains both fibrous and glandular elements. These tumors occur in young women, most commonly ages 15 to 35. Multiple lesions may develop. Growth may be rapid especially at the end of a menstrual cycle and in pregnancy. Older women characteristically have a single, solitary, more slowly growing lesion. Fibroadenomas frequently calcify and may involute after menopause. Occasionally they may develop in a postmenopausal woman after administration of estrogen.
• History. A painless mass is generally discovered by the patient and reported to the physician.
• Physical examination. A well-defined, rubbery, mobile, nontender, 1 to 5 cm mass can generally be palpated. The usual location is in an upper quadrant.
• Diagnostic procedures. A FNAB should be performed or have the patient return in 3 to 6 months for repeat ultrasound and breast examination. Cyroablation may be another option for fibroadenomas confirmed by biopsy. Mammography is not usually helpful, especially in young patients.
• Breast cancer risk. Fibroadenomas are neither cancerous nor premalignant but may require excisional biopsy to confirm the diagnosis.
• Management. Excisional biopsy is both diagnostic and curative.5
• Cystosarcoma phyllodes is a rapidly growing fibroadenoma that recurs if not completely excised. This tumor is rarely malignant, but, because of its extreme size, simple mastectomy may be necessary to achieve complete removal.
NIPPLE ANOMALIES
Nipple Discharge
Nipple discharge is an extremely common concern in young women and most isolated complaints of discharge are of a benign origin. It is practical to divide nipple discharge into two categories based on the presence or absence of galactorrhea. Normal, healthy women commonly have some degree of clear or milky nipple discharge following pregnancy and lactation that can either spontaneously drain from the breast or be produced by palpation. This discharge may be more frequently noted just before menses or with breast stimulation as part of sexual activity. This benign discharge has a small volume, and the amount does not change over time. However, characteristics of pathologic discharge include unilaterality; presence from a single duct; association with an underlying mass; spontaneous, intermittent, and persistent occurrence in a postmenopausal woman; and bloody to serosanguinous color.
• History. History should elicit the nature of discharge, underlying mass, laterality, single- or multiple-duct involvement, relation to menses, color of discharge, the menopausal status of the patient, association with hormonal therapy, and whether the discharge appears spontaneously or must be expressed. The amount and type of nipple stimulation should also be explored. Nipple discharge in a postmenopausal woman is more ominous and is more likely to be caused by cancer.
• Physical examination. A complete breast examination should be performed, with attention to identifying masses, underlying induration, and lymphadenopathy as well as characterizing the discharge. Warm compresses placed on the breast may enhance the ability to detect a discharge. It is important to note whether the discharge originates from one or more ducts. A “pseudo-discharge” is a stain on clothes originating from outside the breast (such as from an abrasion, eczema, or viral condition like herpes). If nipple crusting is present, Paget disease should be excluded by a skin biopsy.
• Fluid characteristics. The characteristics of the fluid may aid in diagnosis. Green, black, creamy, or mucoid discharge is characteristic of fibrocystic breast disease. Straw-colored discharge is most commonly due to a papilloma (which is benign histologically and has only a slight potential for malignant degeneration). Bloody or serosanguinous discharge is associated with malignancy, but may also represent bleeding papilloma or fibrocystic change with an intraductal component. Bloody discharge, or brown-green discharge suggesting old blood, should be investigated further. Cheesy discharge often results from duct ectasia, a chronic inflammatory reaction resulting in permanent distention of the major ducts. The typical patient with duct ectastia is a multiparous woman 40 years or older who notes thick, white, or discolored cheesy material draining from the nipple and noncyclical, burning breast pain. Purulent discharge could indicate an underlying mastitis.
• Diagnostic procedures
• Mammography. A mammogram should be performed in women with abnormal discharge older than 30 years of age and may reveal abnormalities such as the presence of an associated mass.
• Ultrasound. Ultrasound should be performed in conjunction with mammography. It should be directed at the periareolar area to visualize any dilated ducts. Visualization of ductal pathology as small as 0.5 mm can be seen.
• Galactography. The role of galactography and/or ductography requires that iodine be injected into the duct with discharge. Intraductal filling defects, complete ductal obstruction, or an abnormality in the ductal wall may be seen. A negative galactogram does not replace the need for terminal duct excision.
• Magnetic resonance imaging (MRI) and MR ductography. MRI and MR ductography have the ability to provide three-dimensional images of the ducts that are dilated. The role of this technology continues to evolve and is not completely understood yet.
• Fluid analysis. The discharge can be tested for the presence of blood (with a heme occult slide). Gram staining can be performed to identify white blood cells if there is a concern for infection. Fat stain can demonstrate fat globules indicative of milk if galactorrhea is suspected. Cytologic examination of the nipple discharge has no definite benefits.
• Management. Management includes surgical exploration of the duct and removal of the papilloma, if present.3–5
Galactorrhea
Galactorrhea consists of a milky discharge from the breast beyond 6 months postpartum in a non-breastfeeding woman. Although galactorrhea can have many causes, it is usually benign. It has been described in women who jog because friction between the nipple and clothing can stimulate prolactin. Athletic activities may also trigger endorphin release from the hypothalamus, which stimulates prolactin secretion. Correlation is poor between the presence of lactation and serum prolactin level. Galactorrhea is not associated with an increased risk of breast cancer.
• History. History should include recent childbirth, excessive breast stimulation, and medication use. Additionally, it should be determined whether galactorrhea is present from both nipples and from multiple ducts. Galactorrhea from multiple ducts in a nonlactating woman may occur in certain syndromes (Chiari–Frommel, Argonz–Del Castillo). Processes that inflame or irritate the chest wall such as thoracotomy, herpes zoster infection, radiation to the chest wall, burn, may also cause galactorrhea (presumably from a stimulatory increase in prolactin secretion) and should be investigated. Any associated change in menstrual pattern, such as amenorrhea or oligomenorrhea, is suggestive of a central nervous system lesion and approximately 20% of patients with galactorrhea will have a prolactin-secreting pituitary tumor. Headache or visual change may indicate the presence of an intracranial process. Conditions that affect the pituitary and/or the hypothalamus (tuberculosis and multiple sclerosis) and other chronic medical conditions (chronic renal failure, hypothyroidism, Cushing disease) may cause galactorrhea and should be explored in the past medical history.
• Medication history. Medications may be the cause of galactorrhea in up to 20% of patients. Drugs associated with galactorrhea include digitalis, marijuana, heroin, dopamine receptor blockers, phenothiazine, haloperidol, metoclopramide, isoniazid, antidepressants, reserpine, methyldopa, atenolol, cimetidine, benzodiazepines, amphetamines, verapamil, cocaine, progesterones, oral contraceptives, copper-containing intrauterine devices (IUDs), and others. Herbal products that can cause galactorrhea include fenugreek seed, fennel, and red clover. Post–oral contraceptive galactorrhea may occur as well where the milk production is triggered by the withdrawal of estrogen and progesterone. This usually resolves spontaneously. Some patients eventually develop radiologically evident pituitary adenomas.
• Diagnostic evaluation. Serum prolactin level, TSH, and renal function tests can be useful. Further endocrine workup may be indicated. If serum prolactin is greater than 100 ng per mL, brain computed tomography (CT) or MRI is necessary to rule out pituitary adenoma. Non-pituitary prolactin-producing malignancies are less common but include bronchogenic carcinomas, renal adenocarcinomas, Hodgkin disease, and T-cell lymphoma. A CT or MRI scan is necessary if serum prolactin is elevated; if serum prolactin is normal but the patient has an aberration in her menstrual pattern; or if any central nervous system symptoms or signs are present.
• Management. Any medication associated with galactorrhea should be withdrawn. Any thyroid abnormalities detected on workup should be treated. If serum prolactin is elevated, a workup for pituitary adenoma is indicated. If elevated serum prolactin but no pituitary adenoma is demonstrable, treatment may still be indicated to decrease the risk of hyperprolactin-associated osteoporosis. If a microadenoma is present but fertility is not desired, and the risk of osteoporosis does not warrant treatment, patients can be followed without therapy. Microadenomas may regress spontaneously and do not typically transform into macroadenomas. Serum prolactin levels can be followed every 6 months, with repeat CT or MRI every 2 to 5 years. If a macroadenoma is present, therapy is indicated to prevent further growth. Medical management consists of bromocriptine, 2.5 mg per day for 1 week, increased to 2.5 mg bid–tid, or pergolide and cabergoline. Side effects may include nausea, nasal congestion, and postural hypotension. Tumor regrowth may occur following withdrawal of the medication. Bromocriptine can be used to lower prolactin levels to normal to allow fertility and to shrink tumor size preoperatively. Transsphenoidal surgery is an option for large tumors and in patients with macroadenomas who wish to become pregnant. However, surgical success is limited as these tumors frequently recur. Radiation may be an option for patients who are not surgical candidates.5,7
Painful Nipples
Breastfeeding Women
Tenderness of the nipples is a common symptom when breastfeeding is initiated. Proper positioning of the baby is necessary, so that the most cracked or tender portion of the breast is at the corner of baby’s mouth and not aligned with the roof of the mouth or tongue, and correct techniques to “break suction” are essential. Nursing position may be changed. Any engorgement should be treated. Alternate which breast is presented first and begin with the less sore one. Warm or cold compresses and crushed ice applied to nipples before nursing may be beneficial. Milk should be expressed until “let-down” occurs. Avoid petrolatum and zinc oxide. The area can be washed with warm water and can air dry with colostrum applied.
Nipples should be examined for the presence of fissures or local infection. If candidal disease is suspected, treat with topical nystatin or antifungals. Thrush or Candida diaper rash in the newborn or maternal Candida vaginitis should be treated concurrently.
A plugged milk duct can present as a white blister on the nipple following breastfeeding and a hardened area in the breast. Soak the nipple in warm water before next nursing. Gently rub a clean washcloth across the tip of the nipple. As baby nurses, massage behind the hard area to encourage milk expression.
Non-Breastfeeding Women
Nipples can develop painful localized irritation and bleeding in joggers. Small elastic bandages can be applied to the nipple before running or other athletic activities. Emollients or low-dose hydrocortisone cream may ameliorate symptoms.
A unilateral, weeping, ulcerated, irritated nipple is suggestive of Paget disease, especially in middle-aged or older women, and may be associated with an underlying ductal carcinoma. Further evaluation is necessary.1,5,7
Gynecomastia
Gynecomastia has a bimodal distribution. Most boys at puberty develop bilateral gynecomastia, which resolves without treatment within 3 years. It is also common for men in their 50s and 60s to experience breast enlargement. Gynecomastia associated with pain, asymmetry, rapid onset or progression, galactorrhea, and/or erectile dysfunction requires further workup. Association with precocious puberty is also a concerning sign.
Drugs and medications that can cause gynecomastia include an extensive list. Many categories of medications, including antiandrogens, antibiotics, antiulcer drugs, chemotherapeutic drugs, cardiovascular drugs, drugs of abuse, hormones, psychoactive drugs, and some miscellaneous drugs. It is recommended that if a patient has gynecomastia, this list of medications be reviewed and the patient’s medication list removed of potential causative agents if able.
Medical conditions that cause gynecomastia include cancer (testes, liver, bronchiole, stomach, or pancreas, especially the human chorionic gonadotropin (hCG)–producing neoplasms), hyperthyroidism, hypogonadism, cirrhosis, renal failure, severe pulmonary disease, Klinefelter syndrome, testicular feminization, and refeeding after starvation.
Laboratory evaluation includes thyroid function tests, renal and liver function studies; if these are normal, luteinizing hormone, hCG, estradiol, and testosterone should be obtained. If hCG is elevated, testicular ultrasonography and search for other hCG-secreting tumors should be undertaken. If estradiol is elevated, a search for an estrogen-secreting tumor should be undertaken.
Older men develop gynecomastia at an age close to that at which male breast cancer occurs. If a breast mass is felt, a combination of physical examination and FNA can establish the correct diagnosis in the majority of patients. Mammography may add little additional information.1,2,5,7
REFERENCES
1. Andolsek KM, Copeland JA. Benign breast conditions and disease. In: Taylor RB, ed. Family medicine: principles and practice. 6th ed. New York, NY: Springer-Verlag; 2003:895–902.
2. Dickson G. Gynecomastia. Am Fam Physician 2012;85:716–722.
3. Onstad M, Stuckey A. Benign breast disorders. Obstet Gynecol Clin N Am 2013;40:459–473.
4. Amin A, Purdy A, Mattingly JD, et al. Benign breast disease. Surg Clin N Am 2013;93:299–308.
5. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician 2012;86:343–349.
6. Klein S. Evaluation of palpable breast masses. Am Fam Physician 2005;71:1731–1738.
7. Santen RJ, Mansel R. Current concepts: benign breast disorders. N Engl J Med 2005;353:275–285.
|
GENERAL PRINCIPLES
Definition
Breast cancer is formed in the tissue of the breast. Ductal carcinoma develops in the lining of the milk ducts and lobular carcinoma develops from the lobules. Cancer confined to the primary tissue is defined as “in situ” and may become invasive in an estimated one-third of cases.1 Lobular carcinoma in situ (LCIS) is not a true cancer or precancer, but an indicator for increased risk.1 The majority of invasive carcinomas are ductal adenocarcinomas (80%), while infiltrating lobular carcinomas are a minority (up to 15%).2 Inflammatory cancers and Paget disease often have an atypical presentation and are less common (1% to 4%).2
Epidemiology
The global incidence of invasive breast cancer is 22.9%, making it one of the most common invasive cancers in all women, and a leading cause of cancer death worldwide.3
Current estimates in the United States are a lifetime risk of 12.3% or one in eight for women with 40,000 deaths occurring annually. Approximately 79% of new cases and 88% of breast cancer deaths will have occurred in women aged 50 and older. About 1 in 3,000 of all breast cancers occurs in pregnant women, the most common cancer detected in pregnancy which does not change the prognosis.1
Risk factors with a relative risk of >4.0 for breast cancer include (a) female sex (>99% of breast cancer cases), (b) increasing age (65+), (c) biopsy-confirmed atypical hyperplasia, (d) certain genetic mutations such as BRCA1 and/or BRCA2, (e) two or more first-degree relatives with breast cancer diagnosed premenopausally, (f) increasing breast density, and (g) personal history of breast cancer. Risk factors with a relative risk of 2.1 to <4.0 include (a) personal history of breast cancer, (b) one first-degree relative with breast cancer, (c) high-dose radiation to the chest, and (d) high endogenous estrogen or testosterone levels. Other risk factors with lower relative risks (1.1 to 2.0) include (a) age at first delivery (>30); (b) early menarche (<12 years); (c) late menopause (>55 years); (d) nulliparity; (e) no history of breastfeeding; (f) height (tall); (g) recent and long-term use of hormone replacement therapy with estrogen and progestin; (h) obesity (postmenopausal), (i) personal history of endometrial, ovary, or colon cancer; (j) alcohol consumption; (k) higher socioeconomic status; (l) Ashkenazi (Eastern European) Jewish heritage; and (m) diethylstilbestrol (DES) exposure.1
Risk models to predict the relative or the absolute risk of breast cancer consist of BRCA probability tools and Breast Cancer Risk Assessment Models (Gail model). The Gail model of risk assessment is the most validated to predict risk and to direct screening guidelines. While risk factors are important, breast cancer can occur in the absence of known risk factors in nearly 60% of women.4
Breast cancer is a predominantly females disease, with less than 1% seen in males. Risk factors include family history, a higher incidence of genetic mutations involving the BRCA genes, and obesity. Worse prognosis is seen in male breast cancers.1
Classification
The concept of breast cancer as a homogenous disease has evolved into a complex disease state based on molecular subtypes, risk factors, clinical behavior, and response to treatment.5 In addition to the tissue of origin, and the histopathology, breast cancer is classified by grade into low, intermediate, and high, based on the loss of breast cancer cell differentiation using the Nottingham scheme.5 The clinical method of classification is based on tumor size, spread to regional lymph nodes, and the presence or absence of distant metastases known as the TNM staging system.4 Whether the cancer stage is early (stage I, IIA, or T2N1), advanced (T3NO), or metastatic (stage 1V) determines the treatment modalities, prognosis, and risk of recurrence.4 Molecular subtypes are based on the receptor status.2 The presence or absence of estrogen receptors (ERs), progesterone receptors (PRs), or human epidermal growth factor receptor 2 (HER2) defines the immunohistochemistry.2 If all the receptors are absent, the tumor is determined to be triple negative.
DIAGNOSIS
Clinical Presentation
The typical presentation is an asymptomatic mass detected by screening mammography or by breast examination found by the patient or clinician.2 Less common symptoms include breast pain; skin irritation or distortion; and nipple abnormalities such as spontaneous discharge, erosion, or inversion particularly with Paget disease.1 Rarely does a metastatic breast cancer present with signs of secondary spreads such as skin changes and nodules, bone or joint pain, hepatomegaly, or axillary node enlargement, occasionally even prior to a detectable lump in the breast.2
History
The history should include date the lump was found, prior breast problems and biopsies, and risk factor assessment. A detailed family history is essential.1
Physical Examination
The breasts should be examined in the upright and supine positions and inspected for differences in size, retraction or eczematous changes of the skin or nipple, and signs of inflammation and lymphedema. The flat surface of the fingertips should be used to palpate the breast tissue using the vertical strip, three-pressure methods. Characteristics, size, and location of a mass should be noted and constitute the clinical breast examination (CBE). The contralateral breast should be similarly examined. The axillary and supraclavicular areas should be checked for adenopathy. Suspicious masses are generally solitary, discrete, hard, fixed, nontender, and unilateral; however, cancer cannot be excluded based solely on physical examination findings.6
Concerning findings include axillary lymphadenopathy, nipple inversion, or skin changes such as retraction. Rarely if advanced, signs of spread can be evident. A complete physical examination should include a pelvic examination if indicated.5
Imaging
Screening mammography of an asymptomatic woman provides two views of each breast using x-ray and will detect 78% of breast cancers in women with an 83% sensitivity for women over 50. About 17% breast cancers will be missed on routine breast imaging. Breast density is a major confounding factor.7 See Table 13.8-1 for screening guidelines.
Diagnostic mammography is used to evaluate women with signs or symptoms of breast cancer and may include additional views or ultrasonographic imaging. Digital mammography utilizes computer-aided diagnosis and interpretation of mammography. Magnetic resonance imaging (MRI) of the breasts may be used in very high-risk patients, particularly in those with gene mutations. In women with breast implants, a special technique called implant displacement views can assure that breast tissue is not hidden; an MRI may be preferred.8
Tomosynthesis, a three-dimensional x-ray imaging with digital reconstruction, has been approved by the FDA in 2013, and somo-v Automated Breast Ultrasound System (ABUS) was approved in 2012 as an adjunct to mammography.9 To evaluate for metastatic disease, PET, CT, and bone scans are used to detect spread. Clinical and self-breast examinations, along with screening mammograms, have been the mainstay of population screening. Despite the improvement in detection and reduction of breast cancer mortality, routine screening has been recently reviewed to have contributed to over diagnosis and overtreatment leading to several national organizations to revise previous recommendations.10
Currently, the onset and cessation as well as the intervals of screening in all age groups, are increasingly controversial and under review. A consensus has not been reached. In general, women are encouraged to use breast self-awareness and enter an individualized decision based on risk and preference. The use of risk models and decision aids can be helpful but have not been validated for use in the general population.11
Surgical Diagnostic Procedures
Definitive diagnosis of a suspicious mass depends on tissue sampling. Breast imaging should be performed prior to biopsy to avoid possible distortion from hemorrhage. Fine-needle aspiration (FNA) is done to obtain samples from a solid mass for cytology. Core biopsy collects a larger sample size, requires a small skin incision, and, however, has the benefits of higher specificity and the use of immunohistopathology, but slightly lower sensitivity. Stereotactic biopsies utilize imaging for accurate localization and are indicated for deeper masses or for suspicious calcifications seen on a screening mammogram. Lumpectomy or an open excisional biopsy may be indicated when needle biopsies are negative, but the mass is clinically suspicious. Axillary node or the sentinel node biopsies are critical for staging and may be undertaken prior to surgery or more commonly intraoperative.2
Pathologic Findings
Pathology reports from breast biopsies include tumor type, in situ or invasive pathology, and immunohistopathology status. With tumor resection, surgical margins are evaluated and if lymph node sampling was conducted, presence of cancer cells is determined. Nonmalignant conditions comprised the bulk of breast disease and include fibrocystic breast disease, fibroadenomas, hamartomas, and other benign tumors and can often have similar presentation and features.
TREATMENT
Breast cancer requires a variety of treatment options depending on the presenting stage and pathology, the patient’s age and preferences. Early stage breast cancer is treated conservatively in a majority of cases.1 LCIS following a diagnosis with biopsy can be followed with observation alone. Locally advanced and metastatic breast cancer requires a multimodal treatment plan.2
Surgery
The primary treatment for the majority of all breast cancers is surgical resection. Types of surgery consist of varying amounts of removal of involved breast and adnexal tissue and include lumpectomy, quadrantectomy, simple mastectomy, and the modified radical mastectomy.1 The type of surgery is determined by the stage of the tumor and must include the patient’s preference for breast conservation surgery.2
Modified radical mastectomy has replaced the classical radical mastectomy as the most common type of surgery. Axillary and sentinel node biopsies are performed for advanced breast cancer at the time of breast surgery. Sentinel node biopsy has replaced the standard axillary surgery due to reduced morbidity and sequelae. After surgical treatment for breast cancer, the patient may elect to undergo breast reconstruction.1
Radiation
Radiation therapy consists of external beam or brachytherapy and is used after surgical excision and on occasion intraoperatively.1 Radiation is indicated most often with invasive disease to provide loco-regional benefits and reduce microscopic spread.12
Breast conserving therapy is a combination of breast conserving surgery, with mandatory moderate dose of radiation therapy in an attempt to provide better cosmesis in appropriate patients. No change has been found for prognosis.1
Systemic Therapy
Chemotherapy, hormonal therapy, and biologic options in various combinations (depending on the tumor size, histology, hormone receptor status, and stage) are considered systemic therapy. These therapies have contributed significantly to the improved outcomes in breast cancer. Neoadjuvant therapy is used prior to surgery and has been associated with improved outcomes, particularly with late-stage cancers by initiating tumor regression and reducing spread. Surgery is always indicated after completing the therapy independent of the initial outcome or regression. Adjuvant therapy is used following surgery and continued for varying periods of times.1
Chemotherapy consists of combinations of cyclophosphamide, methotrexate, 5-fluorouracil, doxorubicin, epirubicin, and paclitaxel. Multidrug therapy is more effective than single-drug therapy for breast cancer treatment.13 Chemotherapy is indicated generally for stage 2 to 4 cancers and may be the only option in receptor negative disease. Chemotherapy can be withheld in low risk early stage ER+ breast cancer. However, it is superior to hormonal therapy even in receptor positive tumors with advanced disease.14
Adjuvant hormonal therapy is particularly significant in estrogen receptor–positive cancers1 and is determined by the menopausal status.14 Tamoxifen blocks the estrogen receptor and can even be used prior to a diagnosis of cancer in high-risk women as preventative therapy. In postmenopausal women, the aromatase inhibitors (letrozole, anastrozole, and exemastane) are indicated due to ineffectively of tamoxifen, and may be the only neoadjunctive therapy if chemotherapy is contraindicated.14
Trastuzumab (Herceptin) is a targeted monoclonal antibody and offers survival benefit for women with HER2-positive advanced breast cancer, when combined with chemotherapy as adjuvant therapy. Everolimimus suppresses tumor growth and can support hormone therapy.
Recommendations for Breast Cancer Screening for Women with Average Risk |
Organization | Routine mammography | Clinical breast examination |
U.S. Preventative Services Task Force (USPSTF) | Biennial screening mammography women 50–74 (B)a | Evidence of CBE’s additional benefit, beyond mammography is inadequate (I) |
American Cancer Society | Once a year age 40 and older | Every 3 yr women in their 20s and 30s. Once a year starting age 40. |
American Academy of Family Medicine | Routine biennial screening for women 50–74 yr of age | Insufficient evidence |
American College of Obstetrics and Gynecology | Once a year age 40 and older | Every 3 yr women in their 20s and 30s. Once a year starting age 40. |
National Cancer Institute | Every 1–2 yr starting age 40 | No specific recommendation |
aUSPSTF grade evidence: B, Inconsistent or fair evidence; benefits of screening are only moderately greater than the harm. I, evidence that the intervention is effective is lacking, of poor quality or conflicting and the balance of benefits, harms, and costs cannot be determined.
Surgery is the safest treatment, especially early in pregnancy. Radiation and chemotherapy have greater adverse effects intrapartum and postpartum. Third-trimester patients can be observed until delivery and then receive prompt therapy.1
Prognosis and Follow-Up
Prognosis for breast cancer has improved over the past 30 years with a 34% reduction of breast cancer mortality. Prognosis is largely determined by the stage. Developed countries report higher survival rates.3 Early stage offers the best results and the 10-year survival for early stage lesions is 75% to 85%. Death can occur 15 to 20 years later, making posttreatment surveillance imperative for primary breast cancer and secondary cancer. Cancer survivorship is becoming important as mortality due to cancer is decreasing and the increased risk of death due to cardiovascular disease is increasingly being recognized. A multidisciplinary approach is recommended.2
Primary Prevention
Although many of the risk factors are not modifiable, a number of modifiable lifestyle risk factors—smoking, postmenopausal obesity, physical inactivity, and possibly diabetes—have been associated with increased risk. Avoiding weight gain, exercising, smoking cessation, breast feeding, and reducing alcohol have been promoted to reduce up to 20% to 42% of breast cancer.1
Secondary Prevention
Between 3% and 5% of all women who have breast cancer have mutations in the BRCA 1 and 2 genes with a lifetime risk of 50% to 80% of breast cancer. Bilateral prophylactic surgery may be indicated for select women at the highest risk for breast cancer, and appropriate counseling is essential. Prophylactic ovarian and uterine removal is occasionally indicated in for the highest risk cohort. Treatment with tamoxifen or raloxifene can be used as chemoprevention for very select subtypes.1
REFERENCES
1. American Cancer Society. Breast cancer facts and figures 2013–12014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Accessed February 28, 2014.
2. Brufsky A, McGuire K, Leone J. Breast Cancer. First Consult, MD Consult. Web site. http://www.mdconsult.com/das/pdxmd/body/442859946-2/0?type=med&eid=9-u1.0-_1_mt_1014646. Accessed April 8, 2014.
3. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed April 8, 2014.
4. Stopeck AT, Chalasani P, Thompson PA. Breast Cancer. Medscape website. http://emedicine.medscape.com/article/1947145-overview. Accessed April 7, 2014.
5. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–410.
6. Apantaku LM. Breast cancer diagnosis and screening. Am Fam Physician 2000;62:596–602.
7. Oestreicher N, Lehman CD, Seger DJ, et al. The incremental contribution of clinical breast examination to invasive cancer detection in a mammography screening program. Am J Roentgenol 2005;184: 428–432.
8. National Cancer Institute. Fact Sheet. http://www.cancer.gov/cancertopics/factsheet/detection/mammograms. Accessed April 7, 2014.
9. National Cancer Institute. FDA approves Ultrasound imaging system for dense breast tissue. http://www.cancer.gov/ncicancerbulletin/100212/page11. Accessed April 8, 2014.
10. Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammograph. Cochrane Database Syst Rev 6: CD001877. doi:10.1002/14651858.CD001877.
11. Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311:1327–1335.
12. Breastcancer.org. Radiation therapy. http://www.breastcancer.org/treatment/radiation. Accessed April 4, 2014.
13. Taghian A, El-Ghamry MN, Merajver SD. Overview of the treatment of newly diagnosed, non-metastatic breast cancer. http://www.uptodate.com/contents/overview-of-the-treatment-of-newly-diagnosed-non-metastatic-breast-cancer?source=search_result&search=newly+diagnosed+breast+cancer&selectedTitle=2%7E150. Accessed February 28, 2014.
14. Smith IE, Chua S. ABC of breast diseases: medical treatment of early breast cancer. III: chemotherapy. BMJ 2006;332:161–162.
|
GENERAL PRINCIPLES
Diagnosis and management of genital epithelial dysplasia requires mastery of colposcopy, punch biopsy, and endocervical curettage (ECC). The colposcope is essentially a stereoscopic operating microscope combined with a bright-light source. Colposcopy with biopsy seeks to identify patients who may have invasive genital malignancy requiring advanced cancer therapies and women who have premalignant changes, which frequently can be managed with outpatient procedures, such as cryotherapy or loop electrosurgical excision procedure (LEEP). The ultimate challenge for the colposcopist is to distinguish normal from abnormal areas and direct biopsy to allow for histologic interpretation of abnormal areas.1 Recent studies have questioned the diagnostic accuracy of colposcopic punch biopsy,2 and suggest that a liberal approach to biopsy be taken to improve diagnostic yield.
INDICATIONS FOR COLPOSCOPY1
1. Abnormal cervical cancer screening (see Chapter 13.4)
• Cytology (Papanicolaou [Pap] smear) with dysplasia or cancer
• Evidence of high-risk (oncogenic) human papillomavirus (HPV) infection
• Persistent unexplained atypia
• Persistent unsatisfactory cytology3
2. Suspicious visible lesion of the cervix, vagina, or vulva
3. Follow-up of previously treated patients
4. History of diethylstilbestrol exposure
5. Colposcopy highly recommended
• Patients with visible persistent condylomata
• Unexplained vaginal discharge, itching, or bleeding
• HIV-infected women
• Intravenous drug abusers
CONTRAINDICATIONS FOR COLPOSCOPY1
Contraindications usually delay rather than prevent the examination, and include:
• Active gonococcal, chlamydial, or trichomonal infections
• Uncooperative patient
• Heavy, active menses
BASIC CERVICAL COLPOSCOPIC FINDINGS1
• Normal cervical findings
• Squamous epithelium
• Columnar epithelium
• Squamous metaplasia
• Squamocolumnar junction (SCJ)
• Transformation zone (TZ)
• Variants of normal
• Nabothian cysts
• Atrophy
• Pregnancy changes
• Inflammatory or infectious process
• Traumatic changes, clefts, or prior therapy
• Abnormal cervical mucosal patterns, indicating the need for biopsy
• Leukoplakia (a white area prior to application of acetic acid)
• Acetowhite change (a whitening following acetic acid application)
• Punctation (a vessel pattern of small red dots usually within an acetowhite area)
• Mosaic (a vessel pattern with the appearance of chicken wire)
• Atypical vessel pattern (abnormal branching, hairpins, corkscrew patterns)
PATIENT PREPARATION FOR COLPOSCOPY
• Providing informational leaflets may improve patient knowledge and reduce psychosexual dysfunction associated with colposcopy.4
• Playing music during the procedure has been shown to reduce anxiety levels and pain.4
BASIC PROCEDURAL STEPS FOR COLPOSCOPY OF THE CERVIX1
1. Perform a bimanual examination.
2. Insert speculum. Gently blot off excess mucus.
3. Adjust and focus colposcope initially on low power.
4. Apply normal saline to clean the cervix; assess for leukoplakia or other gross lesions.
5. Apply a solution of 3% to 5% acetic acid (i.e., vinegar) to allow for acetowhite changes within areas of dysplasia. Reapply acetic acid every 5 minutes as needed.
6. Colposcopically examine the cervix:
a. Determine whether or not the entire SCJ is visible
b. View with a green light filter to assess for abnormal vasculature
c. Identify areas of abnormality that will require biopsy
7. Lugol iodine solution may be applied to enhance one’s impression of the presence or absence of lesions. Lack of black staining on squamous epithelium implies dysplasia.
8. Perform ECC to evaluate for occult cervical canal disease, particularly when no lesions are visible on the ectocervix or when the examination is inadequate (entire TZ is not visible). ECC is contraindicated in pregnancy.
9. Perform punch biopsies of abnormal areas, starting inferiorly.
10. Apply Monsel’s solution or silver nitrate for local hemostasis.
11. Carefully examine the vagina and vulva, and biopsy abnormal areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree