Figure 4.3-1. An algorithm for the treatment of croup. *, dosage 0.6 mg per kg; ‡, dosage L-epinephrine 1:1,000, see text for dose. (From Skolnik NS. Croup. J Fam Pract 1993;37:168, with permission. Copyrighted 2014. IMNG. 111815:814BN.)
• Humidified air. Provision of humidified air, either by having the parent hold the child in his or her arms in the bathroom at home with the shower turned on to generate steam or by using a croup tent in the hospital may be reasonable; however, studies have shown little efficacy for the use of humidified air in the acute care setting.
• Adrenal corticosteroids. Adrenal corticosteroids should be given to all children with croup. Even in mild cases, benefits include a decrease in hospitalization and intubation rate.1 Dexamethasone, 0.6 mg per kg IM or PO, is effective in decreasing airway obstruction, but it has a slow onset of action and often does not take effect for up to 6 hours. Oral and intramuscular dexamethasone have equivalent efficacies. Alternatively, a single oral dose of prednisolone 1 mg per kg could be considered, but this appears to be somewhat less effective than oral dexamethasone.2
• Nebulized budesonide. Budesonide is a highly potent topical steroid that can be administered by nebulizer. It has a short onset of action and is effective in decreasing inspiratory stridor. It should be considered as an alternative for children who are unable to take oral steroids. Inhaled steroids do not show additive effects when given in conjunction with oral or IM dosing.
• L-Epinephrine (1:1,000) at a dose of 0.5 mL per kg diluted in 3 mL normal saline (maximum doses: <4 years 2.5 mL per dose; >4 years 5 mL per dose) administered by nebulizer can be given to acutely decrease the upper airway obstruction seen in moderate–severe croup. L-Epinephrine has been shown to have equivalent potency and safety compared with racemic epinephrine.3 Racemic epinephrine 2.25% can be given in a dose of 0.05 mL per kg, to a maximum dose for children <10 kg of 0.25 mL and for children >10 kg of 0.5 mL. Epinephrine works through α-adrenergic effects, which lead to mucosal vasoconstriction that results in a temporary decrease in edema of the subglottic region of the larynx. Time of onset of action is less than 10 minutes, and duration of action is less than 2 hours. Treatment is very effective but transient; all children who receive racemic epinephrine must be observed for at least 3 to 4 hours because of the possibility of rebound stridor. Side effects of epinephrine include tachycardia and increased anxiety.
• Heliox is a gaseous mixture of oxygen and helium with a lower density than oxygen alone that reduces the resistance to airflow in narrowed upper airways. Heliox has theoretical benefit for severe cases when hypoxia is present, but more studies are necessary to determine its role in the management of croup.
EPIGLOTTITIS
Clinical Presentation
Epiglottitis tends to occur in children who are older (3 to 7 years) than the croup age group with no history of a preceding upper respiratory infection. The disease involves rapid onset of high fever, stridor, sore throat, dysphagia, and drooling. The child is often toxic in appearance and sitting up in the forward leaning position in an attempt to open the severely compromised airway.
Diagnostic Studies
Lateral neck radiography shows a swollen epiglottis, classically referred to as the “thumb sign.” However, if the clinical presentation of a child suggests epiglottitis, the physician should not waste time getting a lateral neck radiograph. Visualization of the epiglottis should be performed as soon as possible in a controlled setting with facilities available for intubation and tracheotomy. Epiglottitis is confirmed by visualization of a cherry-red swollen epiglottis during intubation. Blood cultures should be obtained as the disease is often accompanied by bacteremia.
Treatment
Treatment is twofold. First, the airway must be secured to ensure adequate ventilation. This is usually accomplished through endotracheal intubation done in a controlled setting where tracheostomy can be performed if necessary. Second, intravenous antibiotics effective against H. influenzae type b should be started (cefuroxime, 75 mg/kg/day divided into q8h, or ceftriaxone, 100 mg/kg/day divided into q12h).
REFERENCES
1. Zoordo R, Sidani M, Murray J. Croup: an overview. Am Fam Physician 2011;83:9.
2. Sparrow A, Geelhoed G. Prednisolone versus dexamethasone in croup: a randomized equivalence trial. Arch Dis Child 2006;91(7):580–583.
3. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing l-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics 1992;89:302.
4. Knutson D, Aring A. Viral croup. Am Fam Physician 2004;69:3.
5. Skolnik NS. Croup. J Fam Pract 1993;37:165.
6. Scolnik D, Coates AL, Stephens D, et al. Controlled delivery of high vs low humidity vs mist therapy for croup in emergency departments. JAMA 2006;295:11.
7. Skolnik NS. Treatment of croup: a critical review. Am J Dis Child 1989;143:1045.
8. Russell K, Wiebe N, Saenz A, et al. Glucocorticoids for croup. Cochrane Database Syst Rev 2004;(3):CD001955.
9. Bjornson CL, Klassen TP, Williamson J, et al. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med 2004;351;1306–1313.
10. Klassen TP, Feldman ME, Watters LK, et al. Nebulized budesonide for children with mild to moderate croup. N Engl J Med 1994;331:285.
11. Klassen TP. Croup. An algorithm for the treatment of croup. Pediatr Clin North Am 1999;46:1167.
12. Mauro RD, Poole SR, Lockhart CH. Differentiation of epiglottitis from laryngotracheitis in the child with stridor. Am J Dis Child 1988;142:679.
13. Johnson DW, Jacobson S, Edney PC, et al. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. N Engl J Med 1998;339;498–503.
14. Tanou K, Kalampouka E, Malakasioti G, et al. Viral croup: diagnosis and a treatment algorithm. Pediatr Pulmonol 2014;49:421–429.
| Reactive Airway Disease in Children |
Airway hyperresponsiveness—the tendency of airways to constrict and cause obstruction due to inflammation—characterizes both asthma and wheezing respiratory illnesses in children. This collection of symptoms generally responds to medications such as bronchodilators and/or steroids. A lack of response should bring about further investigation to determine the cause. Although asthma is reactive airway disease, all reactive airway disease is not asthma.
DIAGNOSIS
History
• The most common symptoms include wheezing, shortness of breath, cough, and chest tightness. Determine whether respiratory distress has occurred previously and associated phenomena. Wheezing may be episodic as when associated with upper respiratory infections or nocturnal or seasonal.
• Associated diseases, including allergic rhinitis, eczema, or food allergies, are common.
• Aggravating factors, such as smoke, animals, exercise, upper respiratory infections, foods, and drugs, should be identified.
• A family history of allergies, eczema, or asthma is common.
Physical Examination
• Obtain vital signs, including respiratory rate, temperature, pulse, weight, and oxygen saturation.
• Observe the child’s color and degree of respiratory distress and anxiety. Fatigue and cyanosis signal a severe attack, and the clinician should be prepared for respiratory failure.
• Stridor indicates an upper airway problem, such as croup or foreign body.
• Check if the wheezing is bilateral and document retractions as well as the ratio of inspiration to expiration (I/E). In children without wheezing or who have normal respirations, check for a posttussive wheeze. Lack of wheezing and presence of a normal chest examination do not exclude asthma. In fact, lack of wheezing may indicate worsening of the clinical situation as the airways become so tight, no air movement can be heard.
• Hydration status should be evaluated.
• A complete ear, nose, and throat examination should be performed with a focus on infections, nasal polyps (pathognomonic for cystic fibrosis [CF]), and signs of allergies such as allergic shiners, atopic dermatitis, or rhinorrhea.
Differential Diagnosis
The differential diagnosis of reactive airway disease is important as treatment for the various conditions is different: possibilities include the following conditions:
• Asthma is characterized by recurrent episodes of diffuse wheezing, dyspnea, and cough and is difficult to diagnose in children younger than 3 years but usually begins before 3 years of age. Cough without wheeze is less often a presenting symptom and should make one consider an alternative diagnosis.
• Bronchiolitis is characterized by the insidious onset of wheezing, tachypnea, hypoxia, and chest wall retractions associated with a 2- to 3-day history of rhinorrhea, cough, and low-grade fever in a child younger than 3 years.1 According to evidence, bronchodilator therapy is not indicated in bronchiolitis; however, for cases caused by respiratory syncytial virus (RSV), there may be some improvement with bronchodilators, and a trial of therapy may be useful.2
• Other conditions may be distinguished by the wheezing pattern. A persistent wheeze present from birth may be associated with a congenital anatomic abnormality of the heart or airways. A persistent illness with wheeze may indicate cystic fibrosis, bronchopulmonary dysplasia, laryngomalacia, agammaglobulinemia, or primary ciliary dyskinesia.
• Infectious causes include pneumonia, sinusitis, croup, and bronchitis. Other causes include mediastinal mass, foreign body aspiration, gastroesophageal reflux and recurrent aspiration, exposure to an irritant, and obstructive sleep apnea.
• Large airway obstruction can be caused by foreign bodies, vascular rings, tracheomalacia, tumors, and laryngeal webs.
Diagnostic Tests
• Reversibility of airway obstruction is diagnostic of asthma and can be evaluated with a trial of epinephrine or adrenergic aerosols. If not reversible, consider a diagnosis other than asthma.
• Complete blood count and chest radiographs are useful when fever is present to evaluate for pneumonia. Chest x-ray is also indicated when congestive heart failure is suspected. Both tests are generally normal in reactive airway disease.
• Pulmonary function tests are usually reliable by age 5 to 6 years and are most useful for monitoring chronic asthma; they are not required for the diagnosis. If done to evaluate cough, provocation with methacholine might be needed.1
• Sinus radiographs, pulmonary function tests, studies for reflux, and specific immunoglobulin E (IgE) antibodies or skin testing (75% of people with asthma have environmental allergies) are indicated for patients whose asthma is resistant to the usual treatment or to evaluate for suspected inciting factors.
• Oximetry is useful for determining severity of respiratory compromise but is not helpful in the differential diagnosis.3 Allergy testing can be done in children over 2 years of age, sweat chloride testing if suspicious of CF. Serum immunoglobulins and viral testing such as for RSV may be helpful also.
TREATMENT
Prevention
Warm-blooded animals should be removed from the home. Exposure to dust mites should be minimized by washing bedding and stuffed animals two times per week in water at least 130°F. Wipe off surface dust frequently using a damp cloth. Carpeting and upholstered furniture should be removed. The humidity level should be kept below 50%. Mattresses and box springs and pillows should be encased in airtight plastic covers with tape over the zipper. Regularly wash damp areas, such as shower stalls, basements, and window sills. Avoid environmental irritants. Do not allow smoking. Do not use woodstoves. Avoid strong odors or sprays. Do not clean when the patient is present. Reduce exposure to infections. Avoid daycare settings if possible, and vaccinate appropriately. If symptoms are severe or systemic and steroids are needed regularly, immunotherapy may be necessary.
Pharmacologic Therapy4
• β-Adrenergic agonists are bronchodilators effective in treating early asthmatic responses and exercise-induced asthma. They are the treatment of choice for acute episodes of bronchospasm and a rescue medication in all classes of asthma. Albuterol and levalbuterol have equal efficacy in treatment. The β-agonists are available as metered dose inhalers (MDIs), nebulizer solutions, oral preparations, and parental preparations.
• Corticosteroids are very potent anti-inflammatory medicines. Anti-inflammatory agents are the most important medicine in chronic recurrent asthma. They reduce inflammation, edema, and mucus secretions and restore β-adrenergic responses. There is no evidence that children will have growth reduction secondary to use of inhaled glucocorticoids over the long term. The topical agents are quickly metabolized and rarely cause systemic symptoms. Inhaled steroids are best given 10 minutes after inhaled β-agonists or are available in combined preparations. Oral doses are absorbed quickly and can be used for acute episodes.
• Cromolyn sodium and nedocromil sodium have few side effects. They are anti-inflammatory medicines and have no bronchodilator effect but inhibit mediator release from mast cells. The dosage is two puffs of MDI three to four times per day or 1 unit dose via nebulizer mixed with a β-agonist. The treatment may be decreased to twice a day with adequate clinical response. They are a nonpreferred alternative monotherapy treatment for mild persistent asthma.
• Theophylline is a bronchodilator whose use has markedly decreased in recent years owing to side effects and lack of an anti-inflammatory component. It has a very narrow therapeutic window, and serum levels must be monitored frequently.
• Anticholinergics function as bronchodilators in most patients with asthma and enhance the effect of the β-agonists. Ipratropium bromide is poorly absorbed and has few systemic side effects. It is particularly useful in cold air–induced, irritant-induced, and emotionally induced asthma.
• Leukotriene modifiers: Montelukast is approved for age 2 and above, zafirlukast is approved for age 5 and above, and zileuton is approved for age 12 and above; however, these are rarely used due to the need to monitor liver functions with these medications. Leukotriene antagonists work on the inflammatory cascade in asthma, are useful in wheezing set off by allergy, and give relief of exercise-induced wheezing. They show some benefit for short-term symptom control and lower respiratory sequelae in infants with RSV.
• Long-acting β-agonists (LABAs) are not to be used as rescue medication or for acute bronchospasm. Salmeterol is approved for age 4 and above and formoterol is for age 5 and above with neither being first-line treatment. Studies suggest that in children an increased dose of steroids may improve airway responsiveness, by reducing inflammation, to a greater degree than the addition of an LABA.5 According to the newest guidelines from the National Heart, Lung and Blood Institute of NIH, inhaled corticosteroids are still the preferred long-term control treatment, but a combination of LABA with corticosteroids is also an equally good treatment option.6
• Influenza vaccine is useful in preventing virus-induced episodes of asthma or reactive airway wheezing secondary to inflammation. It is now recommended for children starting at age 6 months and repeated yearly.
Management
“Step-care” management strategy of asthma, in which the number of medications and frequency of use are increased as symptoms worsen, is recommended by the National Heart, Lung and Blood Institute.4 Treatment of comorbid conditions will benefit management. Severity is best determined when not on a long-acting controller medication, but if already on one, the ability to maintain control will be the determining factor. When determining management success or need to increase use of medication, it is important to assess patient adherence, including correct use of medication both in method and timing.
• Mild intermittent asthma
• Long-term control—No daily medication needed.
• Quick relief—Short-acting bronchodilator; inhaled β-agonists as needed.
• Mild persistent asthma
• Long-term control—Daily anti-inflammatory: low-dose inhaled corticosteroid.
• Cromolyn or nedocromil could be used as a second choice.
• Quick relief—Short-acting bronchodilator; inhaled β-agonists as needed.
• Leukotriene modifiers can be considered in this group as an alternative, but not all are responders to this medication.
• Moderate persistent asthma
• Long-term control—Daily anti-inflammatory: inhaled corticosteroid, medium dose, or inhaled corticosteroid low-medium dose with long-acting bronchodilator, especially for nighttime symptoms. If needed: anti-inflammatory: medium–high-dose inhaled corticosteroids and long-acting bronchodilator, especially for nighttime symptoms.
• Quick relief—Short-acting bronchodilator; inhaled β-agonists as needed.
• Severe persistent asthma
• Long-term control—Daily anti-inflammatory: high-dose inhaled corticosteroid and long-acting bronchodilator. Oral corticosteroid tablets or syrup (2 mg/kg/day; not to exceed 60 mg/day) may be required intermittently.
• Quick relief—Short-acting bronchodilator; inhaled β-agonists as needed.
Flowmeters
Peak flowmeters, which measure peak expiratory flow rate (PEFR), are essential to manage asthma properly but not useful in very young children due to their inability to perform the exercise properly. They also are not useful for diagnosis. The following are interpretations of flowmeter readings.
• The green zone is defined as a PEFR of 80% to 100% of personal best: No symptoms are present; the patient can engage in full activity, and no change in medication is needed.
• The yellow zone is defined as a PEFR of 50% to 80% of personal best: The patient is at increased risk for asthma attacks; treatment should be applied per the step-care management strategy (see above).
• The red zone is defined as a PEFR of less than 50%. Call the physician; emergency care is necessary.
Indications for Admission
Indications include continued wheezing an hour after administration of β-agonist in association with any sign of respiratory distress, persistent tachypnea, PCO greater than 40, PaO2 less than 70, O2 saturation less than 92%, PEFR less than 40% of predicted or personal best, or altered level of consciousness.
Indications for Consultation
Required if multiple hospital admissions, continuation of symptoms, PEFR less than 90% of predicted and never returning to baseline, and poor status following intubation, repeated use of oral corticosteroids to maintain control, additional testing that would be done only by a consultant, or treatment with immune modulators.
Resources for Patient Education
• National Asthma Education Program, DHHS, Publication No. 10-7541, 2010.
• American Lung Association, (800) 586-4872, www.lungusa.org.
• Asthma and Allergy Foundation of America, (800) 727-8462, www.aafa.org.
• Allergy and Asthma Network/Mothers of Asthmatics, Inc., (800) 878-4403, www.podi.com/health/aanma.
• National Asthma Education and Prevention Program, (301) 251-1222, www.nhlbi.nih.gov/nhlbi/nhlbi.htm.
• American Academy of Allergy, Asthma, and Immunology, (800) 822-2762, www.aaaai.org.
REFERENCES
1. Barcy TL, Graber MA. Respiratory syncytial virus infection in infants and young children. J Fam Pract 1997;45:473–481.
2. King VJ, Viswanathan M, Bordley WC. Pharmacologic treatment of bronchiolitis in infants and children: a systemic review. Arch Pediatr Adolesc Med 2004;158:127.
3. Stemple DA, Redding GJ. Management of acute asthma. Pediatr Clin North Am 1992;39:1311.
4. Covar RA, Spahn JD. Practical guide for the diagnosis and management of asthma. National Asthma Education Program; 2010. DHHS Publication No. 10-7541.
5. Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. N Engl J Med 1997;337:1659.
6. National Heart, Lung and Blood Institute. Highlights of major changes in EPR-3: Full Report 2007; 2.
7. Covar RA, Spahn JD. Treating the wheezing infant. Pediatr Clin North Am 2003;50(3):631–654.
8. Strunk RC. Defining asthma in preschool-aged children. Pediatrics 2002;109:357–360.
|
GENERAL PRINCIPLES
Viral exanthems of children are common clinical problems encountered by the family physician. Numerous viral agents can produce a similar rash and other clinical symptoms, which makes the diagnosis challenging. A careful evaluation that includes age, immunization status, history of infectious diseases, exposures, medication use, prodromal period, features of the rash, fever, and the presence of pathognomonic signs is helpful in establishing a diagnosis. Laboratory testing may be available to confirm the diagnosis but often is not acutely useful due to the time delay in obtaining viral cultures or serologic antibody titers. The availability of polymerase chain reaction (PCR) and other nucleic acid–based testing has improved the ability to diagnose many infectious diseases, but these tests are expensive and often do not change management. Treatment in most cases is supportive.
MEASLES (RUBEOLA)
• Causative agent. Measles is caused by an RNA paramyxovirus that belongs to the genus Morbillivirus. Infected individuals are contagious up to 7 days after the onset of symptoms.
• Clinical manifestations may initially include fever, cough, conjunctivitis, and Koplik spots (an enanthem). Rash appears on the third or fourth day and begins as a purple–red maculopapular eruption along the hairline, forehead, and face. By the third day, it spreads to the feet and it fades in the order of appearance. Acute encephalitis occurs in 1 to 2 per 1,000 reported cases and may be fatal.
• Diagnosis. This is usually made on clinical grounds, but viral cultures of body fluids or serologic testing is confirmatory. Serology is the most common method of laboratory confirmation with detection of specific IgM in a specimen of serum diagnostic of acute infection. Acute infection can also be confirmed with a fourfold or greater increase in IgG antibody concentrations between acute and convalescent sera.1
• Treatment of uncomplicated infections is supportive. There is no specific antiviral therapy; however, vitamin A is effective and the WHO recommends administration of once-daily doses of 200,000 IU of vitamin A for 2 consecutive days to all children aged 12 months or older who have measles to reduce morbidity and mortality.1
RUBELLA (GERMAN MEASLES)
• Causative agent. Rubella is caused by a single-stranded RNA virus of the family Togaviridae. Infection is acquired via inhalation of infectious large particle aerosols, augmented by close contact with infected individuals.
• Clinical manifestations may include low-grade fever, postauricular adenopathy, headache, and myalgias. Rash consists of pink–red maculopapules that are discrete and do not coalesce. They appear first on the face and spread rapidly downward to the neck, arms, trunk, and lower extremities. The total duration is 3 to 4 days, with occasional brawny desquamation. Complications are rare but may include joint manifestations, thrombotic thrombocytopenic purpura, and encephalitis. Most adults are immune to rubella, but infection during pregnancy is associated with pre-term delivery and potential for congenital problems, including cardiac and neurologic disorders.
• Diagnosis is clinical, but this can be unreliable. Specific IgM antibody can be detected 4 days after the onset of rash and is usually detectable after primary infection for 6 to 8 weeks. Rubella infection and reinfection can be determined by a fourfold rise in rubella IgG antibody titers between acute and convalescent sera. PCR testing to detect rubella RNA is available but rarely necessary.
• Treatment is symptomatic and supportive. Nonimmune adults should receive a measles, mumps, and rubella (MMR) booster vaccine, especially if future pregnancy is anticipated.
ROSEOLA INFANTUM (EXANTHEM SUBITUM; SIXTH DISEASE)
• Causative agent. Roseola is caused by the human herpes viruses type 6 (HHV-6) and type 7 (HHV-7). Peak prevalence is 7 to 13 months and the majority of cases occur in children younger than 2 years.3
• Clinical manifestations include abrupt onset of fever, commonly 40°C to 40.6°C, which persists for 3 to 5 days with a rapid decline. Other symptoms include fussiness, rhinorrhea, and cough. Rash appears after defervescence. The lesions are pink macules or maculopapules 2 to 3 mm in diameter that blanch with pressure. They appear first on the trunk and then spread to the neck, face, and upper and lower extremities. The total duration is 1 to 2 days.
• Diagnosis is clinical. Serologic testing for HHV-6/7 IgM and IgG is available. Viral cultures from blood and DNA-PCR assays are available but rarely necessary.
• Treatment is symptomatic and supportive including the use of acetaminophen for fever.
ERYTHEMA INFECTIOSUM (FIFTH DISEASE)
• Causative agent. Erythema infectiosum is caused by the human parvovirus B19. Several genotypes from the family Parvoviridae have been identified. It is transmitted through close person-to-person contact and respiratory secretions. It is moderately contagious, with outbreaks occurring in classrooms, daycares, and families.
• Clinical manifestations include fever, headache, and myalgias. Rash has a sudden onset on the face, with marked erythema (“slapped cheek” appearance) with circumoral pallor. This is followed by a generalized lace-like rash on the trunk and extremities that may persist for several weeks. Heat, bathing, sunlight, or local irritation may cause a flare up of the rash. Potential complications include erythrocyte aplasia, arthropathy, and fetal hydrops.
• Diagnosis is clinical but may be confirmed by serologic testing for parvovirus IgM antibody. IgG antibody is present at 15 days postinfection and may be helpful for determining past infection and immune status. Detecting parvovirus B19 DNA using NAAT is now routinely performed by many laboratories but is generally indicated only for complicated cases.
• Treatment of uncomplicated cases is supportive. Intravenous immune globulin has been used in patients with chronic infections and immune deficiency.4
ENTEROVIRAL INFECTIONS
• Causative agent or agents. The enteroviruses consist of numerous strains of echoviruses, Coxsackie viruses, and polioviruses. Incidence of these infections is greatest in the summer and fall.
• Clinical manifestations are varied and include fever, gastroenteritis, respiratory disease, meningitis, and myocarditis. Rash consists of a variety of exanthems that are generalized, maculopapular, and nonpruritic but are not distinctive enough to make a specific diagnosis. Petechial lesions may be seen with type 9 echovirus and group A Coxsackie virus. Hand, foot, and mouth disease is characterized by vesicular stomatitis and cutaneous lesions of the distal extremities. Duration of the infections varies with age and viral type, lasting from a few days to 2 weeks.
• Diagnosis is clinical but may be difficult and often becomes one of exclusion. Although not usually necessary, laboratory diagnosis of enterovirus infections can be done by cell culture of virus, detection of enterovirus RNA with PCR testing, or by serology with acute and convalescent titers.
• Treatment of uncomplicated cases is symptomatic and supportive. There are currently no vaccines or specific antiviral agents for treatment of enteroviruses.
KAWASAKI DISEASE (MUCOCUTANEOUS LYMPH NODE SYNDROME)
• Causative agent. The etiology of Kawasaki disease (KD) remains unknown. Approximately 85% of children with KD are under the age of 5 years, with peak incidence at 18 to 24 months.5
• Clinical manifestations include fever (≥5 days), nonpurulent conjunctivitis, erythema and fissuring of the lips, induration of the hands and feet, enlarged lymph node mass (>1.5 cm), and rash. The rash is deeply erythematous and polymorphic and most commonly manifests as pruritic plaques that vary from 2 to 10 mm. They may resemble urticaria or the target lesions of erythema multiforme. Distribution is variable and may be diffuse, truncal, or limited to the extremities. It slowly fades, and desquamation may occur with resolution of clinical illness. Coronary artery (CA) aneurysms occur as sequelae of vasculitis in 15% to 25% of untreated children, and 2% to 3% die as a result of coronary vasculitis.
• Diagnosis is clinical and must include five of the six clinical manifestations mentioned above. Children suspected of having KD but not having all diagnostic criteria may have “incomplete” or atypical KD. They are treated the same as patients with classic KD.
• Treatment includes intravenous immune globulin (2 g per kg as a single dose) and aspirin (30 to 50 mg/kg/day) until afebrile for 48 hours and then decreased to 3 to 5 mg/kg/day until markers of acute inflammation (erythrocyte sedimentation rate, C-reactive protein, platelet count) normalize. Aspirin therapy can be stopped within 2 months in children with no CA abnormalities detected by echocardiography.
INFECTIOUS MONONUCLEOSIS IN CHILDREN
• Causative agent. Mononucleosis, usually caused by Epstein–Barr virus (EBV), occurs in children but is most commonly seen in adolescents and young adults (see Chapter 19.3).
• Clinical manifestations usually include fever, tonsillopharyngitis, cervical lymphadenopathy, splenomegaly, and fatigue. The rash with EBV infection occurs in 10% to 15% of patients and is generalized and maculopapular or urticarial. A rash often occurs following inappropriate prescribing of ampicillin, amoxicillin, or other antibiotics.
• Diagnosis is made by serologic testing for EBV antibody. Rapid tests for heterophile antibodies (Monospot) are used to screen patients for infectious mononucleosis. These tests are negative in 25% of patients during the first week of infection and in 10% during or after the second week. If clinically necessary, a definitive diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against viral capsid antigens, early antigens, and EBV nuclear antigen proteins.6
• Treatment is symptomatic and supportive for uncomplicated cases.
PRIMARY VARICELLA (CHICKENPOX)
• Causative agent. Varicella is caused by the varicella-zoster virus (VZV) and is one of the most contagious of childhood viral illnesses.
• Clinical manifestations include fever, headache, and malaise. Rash is characterized by the rapid evolution of macule to papule to vesicle. The vesicles, which resemble dewdrops, are 2 to 3 mm in diameter, pruritic, and rupture easily. The lesions appear in crops involving the face, extremities, and trunk. A unique feature of the rash is that the lesions in all stages may be found in the same anatomical area. They crust over by day 7 to 10.
• Diagnosis is clinical. Direct immunofluorescence testing can provide results in several hours. PCR also allows identification of VZV from skin lesions and other specimens. IgM serology for rapid diagnosis has not been demonstrated, and IgM indicated immunity or prior infections. Culture of VZV has a low yield and requires prolonged incubation time.
• Treatment is usually symptomatic and may include antipyretics and antihistamines. Oral or intravenous acyclovir may be used in complicated cases or for immunocompromised children. The live-attenuated two-series VZV vaccine is highly effective in preventing primary varicella although breakthrough cases do occur but are usually mild compared to primary infection.7
REFERENCES
1. Moss WJ, Griffin DE. Measles. Lancet 2012;379:153–164.
2. Best JM. Rubella. Semin Fetal Neonatal Med 2007;12(3):182–192.
3. Zero DM, Meier AS, Sleek SS. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005;352:768–776.
4. Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–597.
5. Eleftheriou D. Management of Kawasaki disease. Arch Dis Child 2014;99:74–83.
6. Luzuriaga K. Infectious mononucleosis. N Engl J Med 2010;362:1933–2000.
7. Baxter R. Long-term effectiveness of varicella vaccine. Pediatrics 2013;131:1–8.
| Common Musculoskeletal Problems of Children and Adolescents |
Musculoskeletal conditions in children and adolescents represent a significant percentage of visits to physicians and emergency departments, contributed to by the higher incidence of fractures, other traumatic injuries, and overuse syndromes from youth sports activities as well as from free play. Prevention in many of these issues is paramount. This chapter provides an overview of a select number of important musculoskeletal conditions, not including acute fractures.
JUVENILE IDIOPATHIC ARTHRITIS
Juvenile idiopathic arthritis (JIA) is an umbrella term for a group of common arthritic conditions that constitute the most common form of childhood arthritis and also one of the more common chronic conditions in children. As the clinical course, immunogenetic associations, and the outcomes are most often quite different from patients with adult-onset rheumatoid arthritis, the JIA classification has replaced the older Juvenile Rheumatoid Arthritis (JRA) terminology. The etiology for JIA is unknown, and the diagnosis is made by consideration of factors in the history, the physical examination, and laboratory testing. Diagnostic criteria include onset before the 16th birthday, objective arthritis (i.e., swelling, effusion, tenderness, limitation or pain with range of motion, joint warmth) in one or more joints that persists for at least 6 weeks, and exclusion of other causes of childhood arthritis. JIA can be subdivided into seven categories, each with its own unique clinical presentations, immunogenetic associations, complications, and outcomes. No specific laboratory or imaging test establishes the diagnosis of JIA. Most JIA patients do not achieve remission and require long-term treatment. Nonsteroidal anti-inflammatory drugs (NSAIDs) are most commonly used; the use of systemic corticosteroids in children should be minimized. The use of newer therapies such as methotrexate and biologic-modifying medications has contributed to improved outcomes in JIA.
SCOLIOSIS
Scoliosis is a lateral and rotational spinal curvature greater than 10 degrees and is present in 2% to 4% of adolescents at the end of their growth period. The majority of curves are due to idiopathic scoliosis and emerge in adolescence (age 10 to skeletal maturity), whereas infantile scoliosis (before age 3: less than 1% of cases) and juvenile scoliosis (age 3 to 10: 12% to 21% of cases) are much less common. Severe curves (greater than 100 degrees) are uncommon, are more likely to have an infantile or juvenile onset, and may lead to significant restrictive pulmonary disease and a shortened life expectancy. Secondary forms of scoliosis are caused by inherited disorders of connective tissue (e.g., Ehlers–Danlos syndrome, homocysteinuria, Marfan syndrome), neurologic disorders (e.g., syringomyelia, tethered cord syndrome, spinal tumors, neurofibromatosis, upper and lower motor neuron disease), and musculoskeletal conditions (e.g., herniated disc, osteogenesis imperfecta, spondylolysis, developmental dysplasia of the hip (DDH), leg length discrepancy, Klippel–Feil syndrome).
Idiopathic Adolescent Scoliosis
This is the most common spinal deformity evaluated by primary care physicians, present in 2% to 4% of the general population, and curves greater than 10 degrees are more common in females. The etiology is believed to be multifactorial, with a strong familial predisposition, thought to be a multigene dominant condition with variable phenotypic expression. The most common curve pattern is a right thoracic apex (90% of thoracic curves), followed by right thoracic–left lumbar, thoracolumbar, double thoracic, and left lumbar curves. Patients with other curve patterns or curves associated with pain or stiffness that are likely due to underlying pathology should undergo expedient evaluation.
• Clinical presentation. Children generally present with cosmetic concerns or are detected during routine physical examinations. Red flags for secondary causes include a left thoracic curvature, significant pain, and neurological symptoms or abnormalities on examination. Most school-based screening programs for scoliosis, commonplace in the past, have been discontinued on the basis of U.S. Preventive Services Task Force (USPSTF) recommendations and concerns for over-referral. However, physicians should remain alert for large spinal curves and other red flags when examining adolescents.
• Physical examination. The physical examination reveals varying asymmetries in shoulder and iliac crest height, asymmetrical scapular prominence, and a flank crease. Curves are deemed as “right” or “left” based on their convexity, and named for the location of their apex vertebrae. The Adam forward bending test is the most sensitive and should reveal a right thoracic and possibly a left lumbar prominence. The neurologic examination and gait should be normal. Height measurements with the patient sitting and standing can be repeated every 3 to 4 months to monitor the growth spurt and gauge risk of progression. A scoliometer may be useful for follow-up and reassurance for the patient and family, but can be difficult to standardize and therefore somewhat unreliable.
• Imaging. Patients with a scoliometer reading greater than 5 degrees or who are otherwise suspected of a significant curve can be screened with a single, standing, 36-inch posteroanterior (PA) radiograph. The vertebral levels with the greatest tilt are identified and measured by the Cobb method (the angle between intersecting lines drawn perpendicular to the top of the most tilted vertebrae above the apex and the bottom of vertebrae below the apex is the Cobb angle). Magnetic resonance imaging (MRI) should be considered in patients with onset of scoliosis before age 8, an unusual curve pattern (e.g., left thoracic), rapid curve progression (more than 1 degree per month), neurologic symptoms or deficit, or significant pain.
Risk Factors for Progression
• Spinal growth correlates with ossification of the iliac apophysis from anteriolaterally to posteriomedially. Risser grades of 0 to 2 (Table 4.6-1) are associated with an increased risk, and patients closer to skeletal maturity (i.e., Risser grades 3 to 4) have a somewhat lower risk of progression. Assessing the epiphyseal status on wrist radiographs can also be used.
The Risser Grading System for Spinal Maturity |
Grade 0: No ossification
Grade 1: Ossification of 0%–25%
Grade 2: 26%–50%
Grade 3: 51%–75%
Grade 4: 76%–100%
Grade 5: Complete bony fusion of the apophysis
• Girls, especially between the onset of the pubertal growth spurt (age 10 to 12) until cessation of spinal growth (Risser 4), are at the highest risk for progression of scoliosis. On average, a girl with scoliosis generally has a relatively higher risk of progression before age 12, and a relatively lower risk after age 12.5. Girls generally have a tenfold higher risk of progression than boys.
• Clinical markers of maturity, such as Tanner staging or age at menarche, are important in the evaluation. Peak curve progression occurs during Tanner stage 2 or 3. Delayed puberty and menarche are risk factors for progression. Hypoestrogen status delays maturation of osseous growth centers and allows an accentuated curve.
• Thoracic curves or curves with their apex at a higher vertebral level are at greater risk.
• Overall risk of progression: >10 degrees: 2% to 3%; >20 degrees: 0.3% to 0.5%; >30 degrees: 0.1% to 0.3%; >40 degrees, <0.1%.
Treatment
• Curvature of 0 to 10 degrees is normal.
• For curvature of 10 to 15 degrees, follow up every 6 months for clinical recheck with forward bending test and scoliometer test to check for progression.
• For curvature of 15 to 20 degrees, repeat radiographs every 3 to 4 months in a growing child with a larger curve. For smaller curves or near the end of growth, repeat radiographs in 6 to 8 months.
• For curves greater than 20 degrees, refer to an orthopedic subspecialist for consideration of close (e.g., every 6 months) follow-up, bracing, and surgical options. Bracing can be considered with curves of 25 degrees or more, and often for curves of 29 degrees and higher, particularly in patients lacking skeletal maturity. Spinal surgery with instrumentation (rod placement and bone grafting) is generally reserved for curves greater than 40 to 45 degrees in patients with growth remaining. Modern surgery is accompanied by spinal cord monitoring using somatosensory and motor-evoked potentials, minimizing complications.
SPONDYLOLYSIS AND SPONDYLOLISTHESIS
While back pain in youth in adolescents is fairly common, and there are many causes, primary care physicians should maintain a high index of suspicion for spondylolysis and spondylolisthesis in young athletes who present with back pain. Spondylolysis is a bony defect of the vertebral pars interarticularis. It is generally considered to be a stress fracture due to repetitive lumbar hyperextension and is the most common fourth and fifth lumbar vertebral levels. The pars defect may also be congenital, and is present in 5% to 6% of North Americans and more than 50% of Alaskan Native Americans or in those with a family history of spondylolysis. It is four times more common in gymnasts than in the general population, and should be considered in dancers, divers, cheerleaders, weightlifters, volleyball players, and football lineman, among other athletes, who present with back pain. Nonathletes may be genetically predisposed to pars breakdown with minimal stress, whereas athletes likely place undue stress on a normal pars. In up to one-third of athletes with back pain, spondylolysis is the cause. Spondylolisthesis is anterior displacement of the cephalad vertebral body on the caudad one, and may be related to a history of spondylolysis, where bilateral defects allow the forward slippage. Grade I spondylolisthesis is displacement of 0% to 25%; grade II, 25% to 50%; grade III, 50% to 75%; grade IV, 75% to 100%; and grade V indicates slippage greater than 100%, which means no overlap of the two vertebral bodies.
Clinical Presentation
Low back pain develops in late childhood and early adolescence and is generally mild. Those with signs of inflammatory back pain, including prolonged morning stiffness, where pain is improved by exercise, and unrelieved by rest may in fact have ankylosing spondylitis (those who are HLA B27 positive have a 20% risk; see Chapter 15.2). In contrast, with spondylolisthesis or spondylolysis, back pain is aggravated with standing and activities requiring lumbar hypertension, such as gymnastics, weightlifting, football (linemen blocking), volleyball (serving), cheerleading (tumbling), and ballet. The pain is typically relieved with rest, and is midline or slightly lateral and may be referred to the buttocks or thighs. Radicular pain is unusual except in severe subluxations (i.e., grade III slips or greater.)
Physical Examination
Patients may have a stiff-legged gait due to tight hamstrings. Excessive lumbar lordosis is often present, and there may be tenderness of the lumbar paraspinous muscles, or other evidence of somatic dysfunction (tissue texture abnormalities, asymmetry, decreased intersegmental range of motion, tenderness) at the affected level. Forward flexion does not aggravate the pain, whereas back hyperextension does. In the single-leg hyperextension (“stork” test) test, the patient stands, grasps one knee, and hyperextends the low back. Back pain on the weight-bearing side suggests an ipsilateral pars interarticularis defect.
Radiographs
The initial diagnostic workup for young athletes with back pain of 3 or more weeks duration, when indicated, should include PA, lateral, and right and left oblique radiographs of the lumbosacral spine. The most common site of involvement is between the fifth lumbar and first sacral segment. The pars interarticularis is best visualized on the oblique views, which show a lucent or sclerotic line known as the “collar of the Scottie dog.” The lateral view demonstrates the amount of subluxation in spondylolisthesis. Diagnosis of early spondylolysis with plain radiographs may be difficult because the pars interarticularis may not have completely fractured; this “Scotty dog” may not be readily apparent. If radiographs are normal and suspicion remains, a bone scan or single-photon emission computed tomography (SPECT) scan is indicated. SPECT is the most sensitive test and should be done if the plain bone scan is normal. CT scans are also highly specific. MRI inadequately visualizes the pars in up to one-third of cases and should not be relied on to rule out the diagnosis, but can be beneficial to detect the entrapment and direct impingement of associated spinal nerve roots.
Treatment
• Spondylolysis. Any activity that causes pain should be restricted and the patient started on an antilordosis program of rehabilitation (abdominal and back flexion strengthening exercises, hamstring and hip flexor stretching). NSAID medications can be used for pain. If pain persists in spite of conservative treatment, the patient can be placed in an antilordosis corset or brace, such as a Boston overlap brace with 0 degree lordosis. The brace is worn during waking hours or up to 23 hours per day. For the first 2 to 3 weeks, the patient performs only hamstring stretches. After 2 to 3 weeks or when pain subsides, lumbosacral stretches and abdominal strengthening out of the brace is added. Sporting activity while the brace is worn can be resumed when asymptomatic. Bracing can be weaned after 4 months if the individual is pain free with full sporting activity in the brace. The brace is tapered off by decreasing wear by 1 hour per day each week. Total bracing time is generally 6 to 9 months. Patients should be followed radiographically every 4 to 6 months for possible progression to spondylolisthesis. Most patients respond to conservative management and return to full activity within 6 months of diagnosis. Patients with persistent pain should be referred.
• Spondylolisthesis. Patients with slippage up to 50% can be treated initially similarly to spondylolysis. Patients with slippage greater than 50% or with pain resistant to conservative treatment should be comanaged by an orthopedic or spine surgeon. Fusion surgery is reserved for patients with greater than a grade II slippage and persistent neurologic symptoms.
JUVENILE KYPHOSIS (SCHEUERMANN DISEASE)
Scheuermann disease is an idiopathic condition resulting in anterior wedging of the thoracic vertebrae and a kyphotic deformity greater than 45 degrees. It occurs in approximately 4% to 8% of the population, may be slightly more common in male adolescents than females, and affected individuals are likely genetically predisposed.
Diagnosis
Patients generally present prior to or at the onset of puberty (10 to 13 years) with a concern of a progressive “round-back” or “humpback” deformity occasionally associated with pain. Pain is generally mild and activity related, but is not activity limiting or associated with easy back fatigability. The round-back deformity is accentuated by forward bending but does not fully correct with back extension, which, along with the radiographs, helps to distinguish Scheuermann disease from postural round back. Approximately one-third of patients have associated scoliosis. Excess lumbar lordosis is common and predisposes to spondylolysis at L5 to S1. Severe kyphosis may be associated with cord compression, extradural cysts, thoracic disk herniation, or restrictive lung disease, but these manifestations are rare.
Radiographs
Complete evaluation requires full-length standing anteroposterior (AP) and lateral spine films. The lateral view shows irregularity of the involved vertebral end plates and anterior wedging of three or more contiguous vertebrae by 5 degrees or more. Kyphosis between T4 and T12 measured by the angle of Cobb is greater than 45 degrees. Only one or two vertebral bodies may be involved with thoracolumbar disease. The radiographs should also assess for associated scoliosis, lumbar hyperlordosis, and spondylolisthesis. Lateral hyperextension views are helpful in determining the flexibility of the deformity.
Treatment
• General. Treatment is based on the severity of deformity, presence of pain, and the patient’s age. Curves of 45 to 60 degrees with no evidence of progression are treated with observation, an exercise program to correct lumbar lordosis (abdominal strengthening, increasing hip flexor, and hamstring flexibility), and thoracic spine hyperextension exercises. Recheck every 3 to 4 months.
• Bracing is indicated with significantly painful curves of 50 degrees or greater, progressive deformity, especially in those with an immature skeleton, or for patients with curves that are cosmetically unacceptable.2 A modified Milwaukee brace is most commonly used in conjunction with exercises, is best if initiated before skeletal maturity, and generally requires 12 to 18 months of treatment. Comanagement with an orthopedic or spine subspecialist is warranted.
• Surgery is rarely indicated and reserved for those with severe deformities (generally >75 degrees) or persistent back pain unresponsive to conservative treatment.
BACK PAIN IN YOUTH: RED FLAGS FOR INFLAMMATORY CONDITIONS
Persistent back pain is generally uncommon in childhood—including the above-mentioned conditions. Inflammatory back pain, with onset in adolescence or preadolescence, may reflect an underlying inflammatory condition (e.g., HLA B27–associated ankylosing spondylitis or Reiter syndrome; see Chapter 15.2). Pain in these conditions is typically insidious in onset (over months), not associated with trauma or any visible abnormality described above, low back in origin, worse in the morning, associated with stiffness lasting an hour or longer, improving with exercise, and worsening after sitting.
Physical Examination
• Will sometimes reveal loss of normal lordotic curve (i.e., straightening), associated with decreased forward flexion of the lower 10 cm of the lumbosacral spine. The Schober maneuver measures 10 cm up from a line drawn between the sacroiliac “dimples” when standing fully upright and marks start and end of that distance. The child then bends forward, and the distance between the two marked spots is remeasured. The normal lumbar spine expands from the baseline 10 cm out to 15 cm (expansion of 5 cm). Loss of normal lumbar expansion can indicate an early inflammatory spondyloarthropathy.
• In addition, when hip flexion, extension, and internal and external rotation are normal—but crossing the leg, and pressing down on the knee and opposite pelvic brim (flexion, abduction, external rotation [Faber test]) causes pain in the sacroiliac joint, or the groin—there may be inflammation of the sacroiliac joint.
FLATFOOT (PES PLANUS)
Flatfoot is broadly categorized as either physiologic flexible flatfoot or pathologic flatfoot. Pathologic flatfoot in infants can be secondary to the common but benign calcaneovalgus foot or a more ominous congenital vertical talus. Older children may have a tarsal coalition, hypermobile flatfoot with tight heel cords, or neurogenic flatfoot.
Flexible Flatfoot
• Etiology. The normal arch is not present at birth and slowly develops around 4 to 5 years of age. Excessive laxity of the joint capsule and plantar ligaments allows the developing arch to flatten out while bearing weight. In young children, a fat pad may further obscure the arch.
• Clinical presentation. Children are generally asymptomatic and brought to the physician by the parents with a concern about potential problems related to the flatfoot. The child may have fatigue or aching of the foot muscles with prolonged walking or standing.
• Physical examination. The child’s foot flattens with weight bearing but develops an arch while the child stands on tiptoe or actively dorsiflexes the great toe. Observed from behind, the calcaneus is in valgus position while the child is standing and inverts when the child stands on tiptoe. The child’s ability to stand on the heels indicates adequate heel cord flexibility. The child should be able to stand both on the inner and outer borders of the feet indicating good muscular control and adequate subtalar motion.
• Radiographs. Radiographs are not needed unless other pathology is suspected.
• Treatment. Reassure parents that no treatment is necessary because there is gradual improvement with growth, generally by age 5 years. Arch supports do not generally make a difference in radiographic or clinical outcome. The occasional child who develops symptoms associated with the flatfoot (e.g., foot pain, patellofemoral pain) should be given medial longitudinal arch supports or a medial heel wedge, or both.
PATHOLOGIC FLATFOOT
Pathologic flatfoot is characterized by limited ankle motion and, frequently, foot or ankle pain. Ankle motion may be limited in dorsiflexion by a tight heel cord and in inversion and eversion by subtalar pathology.
Hypermobile Flatfoot with Tight Heel Cord
• Etiology. A tight heel cord combined with a flexible flatfoot forces the calcaneus into a valgus position during ambulation. This compensatory hindfoot valgus allows for more ankle dorsiflexion. The resultant abnormal foot biomechanics lead to pain.
• Clinical presentation. Patients complain of foot or ankle pain.
• Diagnosis. The patient has a flattened arch and calcaneal valgus when standing. Observation from the side shows early heel liftoff during the gait and an arch that develops as the toes dorsiflex. Subtalar motion (calcaneal inversion and eversion) is normal, but ankle dorsiflexion is limited to neutral or less.
• Treatment. Patients with mild symptoms can be initially treated with aggressive heel cord stretching and a medial longitudinal arch support with a medial heel wedge. Those with more severe symptoms can be treated with a short-leg walking cast, with the ankle neutral for 4 weeks followed by heel cord stretching. Surgery for heel cord lengthening and correction of heel valgus may be necessary if conservative treatment fails.
HEEL PAIN
Sever Disease
• Calcaneal apophysitis is common in boys between the ages of 6 and 10, especially in obese children and athletes, secondary to repetitive microtrauma or overuse of the heel and thought to be related to tensile forces from the Achilles tendon on the calcaneal apophysis. It is commonly seen in athletes playing soccer, basketball, track, and other running sports. Pain is usually on the posterior side of the calcaneus, is bilateral in 60% of patients, is more pronounced after activity, and commonly worse at the beginning of a new sports season or during a growth spurt. Tenderness can be elicited at the insertion of the Achilles tendon and on medial and lateral compression of the posterior calcaneus. Acute treatment includes temporary avoidance of high-impact activities, heel lifts or heel cups/cushions, ice massage, and NSAIDs. Once stretching is not painful, adding in calf stretching exercises, other manual therapies and orthotic devices can be helpful. Most young athletes can return to full pain-free activity within 3 to 6 weeks. In refractory cases, cast immobilization may be needed. Patients with persistent symptoms despite adequate rest and a stretching program should lead you to reconsider the possibilities of alternate diagnoses, including Achilles tendonitis, plantar fasciitis, or calcaneal stress fracture.
• Plantar fasciitis. Plantar heel pain that is burning, aching, and occasionally sharp and lancinating and occurs with weight bearing on arising in the morning or after prolonged sitting, often worse for the first few steps taken, is suspicious for plantar fasciitis. This is more common in adults, but can occur in overweight children and adolescents, and risk factors include pes planus, pes cavus, and sedentary lifestyle. Palpation of the medial calcaneal tubercle at the origin of the plantar fascia usually elicits tenderness, as compared with calcaneal stress fracture, where pain is elicited with medial to lateral compression of the calcaneus. Imaging is not routinely indicated initially. Treatment is similar as for Sever disease; relative rest, consideration of orthotic devices that counteract pronation and disperse heel strike forces (e.g., arch supports, heel pads or heel cups), use of NSAID medications in select patients, ice massage and manual/myofacial treatments, and stretching exercises of heel cord and plantar fascia. Using local injections of corticosteroids via a 25-gauge needle can be helpful in resistant chronic cases; extracorporeal shock wave therapy and plantar fasciotomy are reserved for the most recalcitrant and debilitating cases that extend beyond 6 months to a year. Also remember that heel pain, stiffness in the morning, and inflammatory type back pain can be associated with inflammatory spondyloarthropathy.
BOWLEGS (TIBIA VARUS)
Varus angulation of the knee can be normal, secondary to metabolic disease, severe physiologic bowing, or osteochondrosis deformans tibiae (Blount disease).
• Normal development. Children are born with tibia varum, become maximally bowlegged by 6 months, and begin to straighten by 18 to 24 months. Tibia valgum or “knock-knee” develops during the second to third year and peaks by the fourth year. Development then progresses back to the normal adult alignment of slight valgus by age 7 to 8 years. Bowlegs should be fully evaluated if they have not corrected by age 2.
• Metabolic etiology. Parents should be questioned regarding diet, and the child’s growth curve should be reviewed. Rickets, abnormal calcium or phosphorus metabolism, and renal disease should be considered. If a generalized disorder is suspected, screening laboratory tests should be ordered, including serum calcium, phosphorus, alkaline phosphatase, creatinine, and hematocrit.
Severe Physiologic Bowing and Blount Disease
• Clinical presentation. While physiologic tibia varum is a normal variation that the child will outgrow, pathologic tibia varum is referred to as Blount disease. The child typically has painless bilateral tibia varus that is of concern to the parents. Growth and development is otherwise normal. The child may experience pain along the medial side of the knee, and/or trouble walking without tripping.
• Diagnosis. Standing PA radiographs must be obtained while the child’s feet are together or shoulder-width apart and patellae directly forward. A tibiofemoral angle of more than 20 degrees is abnormal.
• Severe physiologic bowing. This is characterized by medial metaphyseal beaking of the distal femur and proximal tibia, medial cortical thickening, and no pathologic changes of the proximal medial tibial epiphysis.
• Blount disease. This disorder is characterized by angulation under the posteromedial proximal tibial epiphysis, tibial metaphyseal irregularity, beaking of the proximal tibia, and wedging of the proximal epiphysis. It is more common in African-American children, where bowing gets worse between the ages of 2 and 4, or it can occur in overweight adolescents.
Treatment
• Severe physiologic bowing. Spontaneous correction generally occurs by age 7 to 8 years. Surgery may be indicated if the deformity persists past age 8.
• Blount disease. Aggressive treatment is needed, with bracing with a hip–knee–ankle–foot orthosis or a knee–ankle–foot orthosis worn 23 hours daily. Surgical (e.g., tibial osteotomy) correction may be needed to prevent permanent damage. Patients should be referred for consideration of surgery once the diagnosis is made or suspected.
INTOEING
General
Intoeing affects a large number of infants and children and is a major source of concern for parents, leading to consultation and questions. Understanding the primary cause of concern is helpful in counseling the parents of the child with intoeing. Knowledge of what is normal and what will self-correct with normal growth and development will prevent unnecessary treatment, identify the rare causes that need intervention, and reassure most parents that the condition will resolve over time with normal growth. The three most common causes are metatarsus adductus, internal tibial torsion, and increased femoral anteversion, and vary in proportion to the age of the child.
Rotational Profile
The parents’ attention focuses on the child’s feet, but the source of intoeing can be anywhere in the lower extremities. Certain definitions are needed to facilitate evaluation of the gait and the lower extremities (Figure 4.6-1).
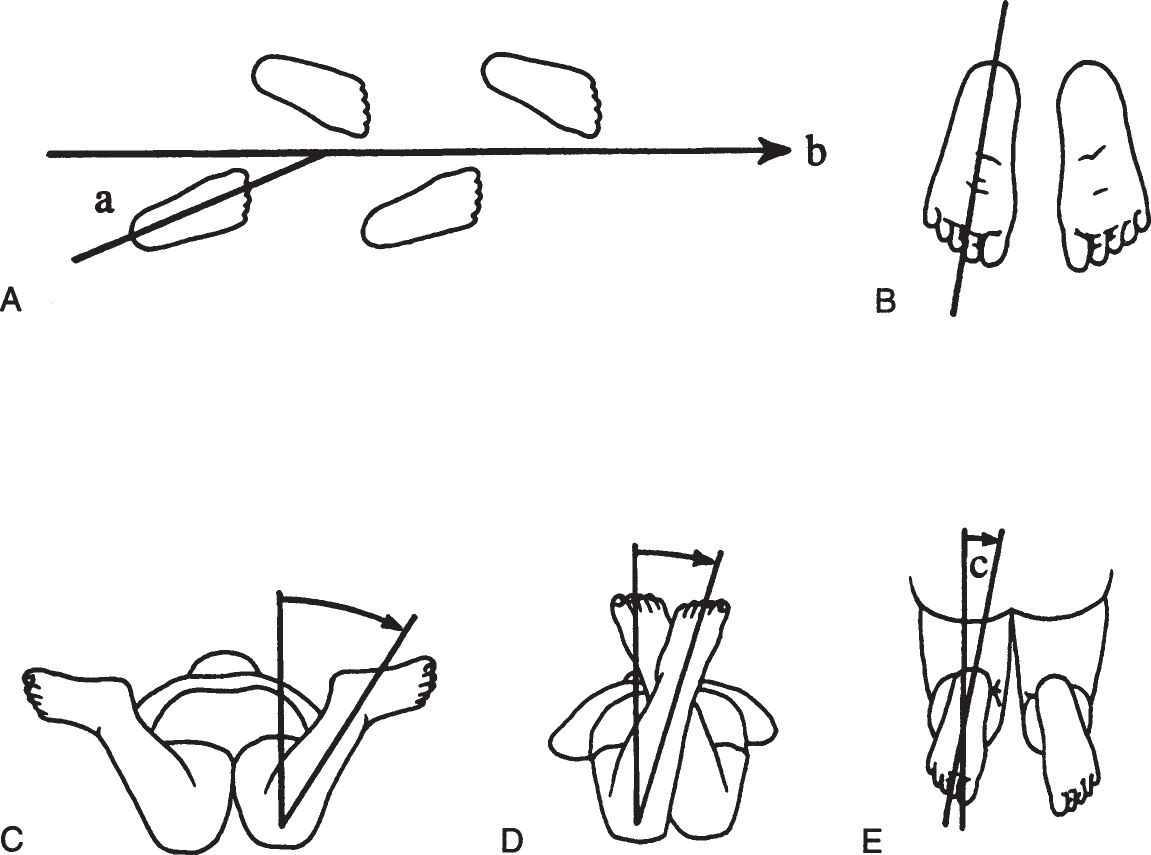
Figure 4.6-1. Rotational profile. A: The angle between the line of progression (b) and the foot axis is the foot progression angle (a). B: Foot axis. C: Internal (medial) femoral rotation. D: External (lateral) femoral rotation. E: Thigh–foot angle (c) is formed by the foot axis and the longitudinal axis of the femur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







