Chapter 116 Probiotics
 Introduction
Introduction
The term probiotic is derived from the Greek and literally means “for life.” It was first coined in 1965 by Lilley and Stillwell to describe substances secreted by one microorganism that stimulate the growth of another.1 In 1974, Parker modified this definition to “… organisms and substances which contribute to intestinal microbial balance.”2 The current World Health Organization definition of probiotics is “live microorganisms which when administered in adequate amounts confer a health benefit to the host.”3 This definition includes fermented foods such as yogurt, sauerkraut, and kefir, as well as specific supplements containing freeze-dried bacteria. The microorganisms found in these products are usually lactobacilli and bifidobacteria.4
Humans have been consuming probiotics for many thousands of years and fermented foods have been, and still are, of great importance to the diets of most of the world’s people. Microbial cultures have been used to produce beer, wine, yogurt, tempeh, sauerkraut, olives, cheese, and many other fermented foods.5 Thus, the symbiotic relationship between humans and probiotic microorganisms has a long history of important nutritional and therapeutic benefits for humans.
 Description
Description
At the turn of the century, Metchnikoff6 asserted that yogurt was the elixir of life. He theorized that putrefactive bacteria in the large intestine produce toxins that invite disease and shorten life. He believed that eating yogurt would cause lactobacilli to become dominant in the colon and displace the putrefactive bacteria. For years, these claims of healthful effects from fermented foods were considered unscientific folklore. However, a substantial and growing body of scientific evidence has now demonstrated that lactobacilli, bifidobacteria, and fermented foods play a significant role in human health.
The genus Lactobacillus is characterized by considerable heterogeneity. Bacteria are classified as lactobacilli if they are gram-positive, nonsporing, and rod-shaped bacteria that produce lactic acid as the major end product of carbohydrate fermentation. Lactobacilli appear to be fairly unique, in that they have been isolated from a number of diverse environments, such as fermented vegetables and dairy foods, as well as the human gastrointestinal tract (GIT) and vagina.7
In contrast, bifidobacteria are not found in natural fermentative processes, but are native to the GIT.8 Bifidobacteria are also gram-positive, nonsporing bacteria; however, they are Y-shaped instead of rod-shaped and their major fermentative end product is acetic acid.9
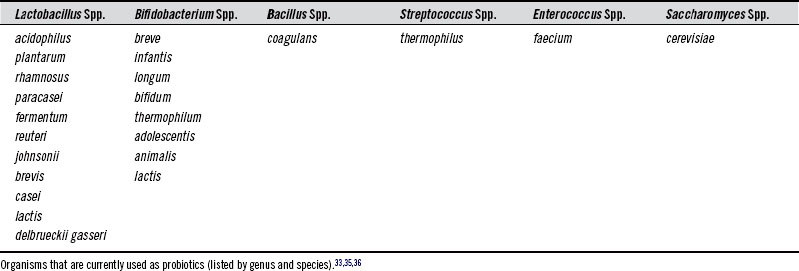
Intestinal colonization by lactobacilli and bifidobacteria begins during the birthing process. Before birth, the GIT of the neonate is completely sterile. During delivery, the newborn is inoculated with microorganisms from the birth canal and the mother’s fecal flora, as well as from organisms in the environment. In the first week, the organisms that are best suited to the intestinal environment become established. Initially, there is often a predominance of Escherichia coli, enterococci, and streptococci. A diet of breast milk creates a colonic environment that favors the growth of a simple flora of bifidobacteria and a few other anaerobes. Breast-milk contains many bifidogenic oligosaccharides,10 as well as living bacteria. Amazingly, breast milk from healthy women can contain up to 109 bacteria/L, including various strains of lactobacilli and bifidobacteria.11–13 In formula-fed infants, the microbiota is more complex (resembling the adult flora), containing far fewer bifidobacteria and more Bacteroides spp., clostridia, and anaerobic streptococci. The introduction of solid food to the breast-fed infant causes major changes to the microflora. A rapid rise in the numbers of enterococci and enterobacteria is followed by increases in Bacteroides spp., anaerobic streptococci, and clostridia. As the amount of solid food increases in the diet, the bacterial flora of formula-fed and breast-fed infants approaches that of adults.14–16 Common species of lactobacilli and bifidobacteria found in the infant and adult human GIT are listed in Box 116-1.11,17–23
 Proposed Mechanisms of Action
Proposed Mechanisms of Action
Many pathogenic organisms must associate with the GIT epithelium to colonize effectively. However, some strains of bifidobacteria and lactobacilli can adhere to the epithelium and act as “colonization barriers” by preventing pathogens from adhering to the mucosa.24 This effect was demonstrated with the Lactobacillus rhamnosus strain GG and L. plantarum 299v. Both of these organisms showed the ability to inhibit attachment of E. coli to human colon cells.25
Another possible mechanism of action is the modification of the microbial flora through the synthesis of antimicrobial compounds. Many types of lactobacilli and bifidobacteria produce bacteriocins or other antimicrobial compounds. Bacteriocins are defined as “compounds produced by bacteria that have a biologically active protein moiety and a bactericidal action.”26 Other biologically active compounds produced by lactic acid bacteria include hydrogen peroxide, diacetyl, and short-chain fatty acids. The release of these compounds by probiotic organisms results in a beneficial modification of the microflora.27 However, not all strains of lactobacilli or bifidobacteria produce antimicrobial compounds, and some produce compounds that are fairly nonspecific in their activity, so that beneficial bacteria, as well as pathogenic organisms, may be negatively affected.
It has also been observed that probiotics can stimulate the immune response. This immune response may take the form of increased secretion of immunoglobulin-A (IgA),28 elevated numbers of natural killer cells, or enhanced phagocytic activity of macrophages.29 Increased secretion of IgA may decrease numbers of pathogenic organisms in the gut, thus improving the composition of the microflora.24,30
Probiotics may also compete for nutrients that would otherwise be utilized by pathogens.31 This situation occurs with Clostridium difficile, a potentially pathogenic organism that is dependent upon monosaccharides for its growth. Probiotic organisms in sufficient numbers can utilize most of the available monosaccharides, which results in the inhibition of C. difficile.32
 Probiotic Characteristics
Probiotic Characteristics
Probiotic organisms require certain characteristics to enable them to exert maximum therapeutic effects. These qualities are summarized in Table 116-1.
TABLE 116-1 The Desirable Characteristics of Effective Probiotic Strains
| CHARACTERISTICS | FUNCTIONAL BENEFIT |
|---|---|
| Human origin | Human origin should translate to ability to survive conditions in the human GIT, as well as the possibility of species-specific health effects. |
| Gastric acid and bile salt stability | Survival through stomach and small intestine |
| Adherence to intestinal mucosa | Believed to be essential for immune cell modulation and competitive inhibition of pathogens. |
| Colonization of intestinal tract | Multiplication in the intestines suggests that daily ingestion may not be needed; immune cell modulation |
| Safety in food and documented clinical safety | Adverse effects absent or minimal; accurate identification (genus, species, strain) |
| Production of antimicrobial compounds | Normalization of GIT flora; suppressed growth of pathogens |
| Antagonism against pathogenic organisms | Prevention of adhesion and toxin production by pathogens |
| Clinically documented and validated health effects | Clinicians can be confident of therapeutic effects; dose–response data for minimum effective dosage in different formulations is known. |
GIT, gastrointestinal tract.
Modified from Mattila-Sandholm T, Salminen S. Up-to-date on probiotics in Europe. Gastroenterol Int. 1998;11(suppl 1):8-16.33
Of these characteristics, there are some that are considered almost essential for a probiotic to have therapeutic effects. These are: (1) gastric acid and bile salt stability; (2) an ability to adhere to the intestinal mucosa; and (3) an ability to colonize the intestinal tract.34 Unfortunately, many commercially available probiotic supplements and yogurts contain strains that do not exhibit these vital characteristics. If a probiotic strain does not exhibit these characteristics, then it will be nowhere near as effective as those that do.
Probiotics in Use
There are many different microorganisms currently used as probiotics. Table 116-2 lists commonly used probiotic species.
To better understand how bacteria are named and classified, the following discussion may be helpful. Genus is the first name of a bacterium (e.g., Lactobacillus). It is somewhat general and refers to a grouping of organisms based on similarity of qualities, such as physical characteristics, metabolic needs, and metabolic end products. Species is a bacterium’s second name (e.g., acidophilus). It is a much more narrow classification based on shared common characteristics that distinguish them from other species. Strain is an even more specific classification that divides members of the same species into subgroups based on several properties that these bacteria have in common that are distinct from other members of the species (e.g., strain LA5).37
Issues in Probiotic Nomenclature
• The species Lactobacillus bulgaricus is now referred to as Lactobacillus delbrueckii ssp. bulgaricus.38
• Lactobacillus bifidus (also known as “bifidus”) was renamed Bifidobacterium bifidum over 30 years ago, yet the improper nomenclature continues to be widely used.38
• Many strains of bacteria that were once classified as Lactobacillus casei have been reclassified as strains of Lactobacillus rhamnosus (e.g., L. rhamnosus GG) or Lactobacillus paracasei (e.g., L. paracasei Shirota strain).39
• Strains of Lactobacillus sporogenes have been renamed Bacillus coagulans (they are not true lactobacilli because they form spores).40
• Bacterial strains that were once classified as Lactobacillus acidophilus (often referred to as “acidophilus”) have now been divided into six species: L. acidophilus, L. gasseri, L. amylovorus, L. gallinarum, L. johnsonii and L. crispatus.39
• Strains of Saccharomyces boulardii are now definitively regarded as a distinct group within the species Saccharomyces cerevisiae.41,42
Importance of Strain
Within each species of bacteria there is a multitude of strains. Some probiotic strains are resilient and strong, able to survive passage through the upper GIT and inhibit pathogenic bacteria, whereas others are weak and cannot survive the upper GIT or kill pathogenic bacteria. It is also important to note that just because one strain of bacteria in a given species has a proven action does not mean that another strain will too, even if they are closely related. Furthermore, actions found in one strain of L. rhamnosus cannot be extrapolated to a strain of L. acidophilus or L. plantarum. Actions and qualities are fundamentally strain specific.43 Therefore, strains of bacteria within the same species can have significantly different actions, properties, and characteristics.
Two strains of L. rhamnosus were utilized in a trial assessing their efficacy in the treatment of viral gastroenteritis. One strain was L. rhamnosus strain GG (LGG); the other was a strain found in a supplemental product (Lactophilus). LGG accelerated recovery from diarrhea, whereas the closely related strain did not.44
Additional in vitro research using two closely related strains of B. bifidum (CIDCA 537 and 5310) found that one strain (CIDCA 5310) inhibited enterocyte invasion by Salmonella arizonae, whereas the other had no effect.45 The results of both these studies demonstrate the principle of strain specificity, that is, different bacterial strains within the same species can have significantly different actions and therapeutic effects.
 Commercial Forms
Commercial Forms
There are two main forms in which probiotic organisms can be ingested—fermented foods and supplements. Fermented foods can be of both dairy and vegetable origin, with the most commonly known of each being yogurt and sauerkraut, respectively. Probiotic supplements consist of freeze-dried (lyophilized) bacteria in powder, capsule, or tablet form. Regardless of the form in which the microorganisms are consumed, for clinical efficacy, products containing probiotic organisms must provide live organisms in sufficient numbers to exert therapeutic effects. Both types of fermented foods and supplements are able to do this. Common probiotic delivery systems are compared in Table 116-3.
TABLE 116-3 The Pros and Cons of Different Probiotic Delivery Systems
| DELIVERY SYSTEM | PROS | CONS |
|---|---|---|
| Fermented dairy | Affordability and easy availability Ease of incorporation into daily patterns Additional nutritional benefits Enhanced bacterial survival through upper GIT (100× less bacteria can be given per dose)63 Effective in the upper GIT | Contains dairy proteins and lactose Taste can be issue Not suitable when travelling Not suitable for vegans |
| Capsules | Ease of administration Contain no binders | Not therapeutic in upper GIT (unless opened or chewed) May contain allergenic excipients Higher cost |
| Tablets | Ease of administration Effective in the upper GIT | May contain allergenic or otherwise problematic binders and excipients (e.g., gluten) Higher cost |
| Powders | Effective in the upper GIT Dosages can be easily adjusted Can be incorporated into foods or drinks Contain no binders |
GIT, gastrointestinal tract.
Fermented Dairy—Yogurt
The origin of fermented dairy products is somewhat obscure, but their consumption is believed to date back to at least 5000 bc.46 Sour milks have always been popular throughout Europe, Asia, and Africa as nutritious, long-lasting foodstuffs. Fermented milks were also considered medicine, with ancient physicians like Hippocrates, Galen, and Avicenna advocating their use for the treatment of gastrointestinal ills.47
Early in the twentieth century, Nobel prize laureate Elie Metchnikoff popularized the idea that fermented milk products could beneficially alter the microflora of the GIT. He attributed the long life of Bulgarian peasants to their consumption of soured milk, which he believed to arrest the abnormal putrefaction of proteins within the bowel.6 Metchnikoff later researched the bacteria found in this Bulgarian milk, Bacillus bulgaricus (now known as L. delbrueckii subspecies bulgaricus) and a type of cocci (now known as Streptococcus thermophilus).47 He utilized these cultures in the manufacture of a type of sour milk he launched in Paris at the beginning of the twentieth century.46
These same species of bacteria are still used today in the manufacture of commercial yogurts. These two bacterial species (L. delbrueckii ssp. bulgaricus and S. thermophilus) are responsible for the taste, consistency, and smell that we associate with yogurt.48,49 It is now known, however, that these species lack the ability to survive in the human GIT. Hence, yogurt manufacturers now routinely add additional probiotic species of bacteria to yogurt in an attempt to enhance its therapeutic effects (e.g., L. acidophilus and B. bifidum).46
The therapeutic efficacy of a specific yogurt depends substantially upon the characteristics of the strains of bacteria that it contains, as well as the number of viable bacteria present at the point of consumption. A therapeutic yogurt will contain bacterial strains with the desired characteristics as outlined in Table 116-1, and these strains should be in sufficient numbers to exert therapeutic effects once consumed (i.e., >106 bacteria/mL of each bacterial strain).50 Recent market-basket surveys showed that some yogurts do achieve and maintain this level of bacterial viability throughout their shelf-life, and furthermore, these same brands of yogurt often utilize bacterial strains with the desired probiotic characteristics.51
A number of studies have attested to the therapeutic efficacy and ability of yogurt and fermented milks to successfully deliver probiotic bacteria to the human GIT.52–62 Yogurt appears to act as an ideal transport medium for probiotic bacteria, as it has been shown to enhance the survival of bacteria through the upper GIT.50 One study found that 108 bacteria given in a milk-base demonstrated greater fecal recovery after oral administration than 1010 organisms given as a freeze-dried powder. Thus, significantly smaller numbers of probiotic bacteria can be given in yogurt than in supplements to achieve similar numbers of viable organisms in the lower GIT.63
Fermented Vegetables—Sauerkraut and Kimchi
Fermented plant foods have always been an important component of the human diet, and are still common food items throughout the world, from sauerkraut in Eastern Europe to kimchi in Southeast Asia. Traditionally prepared, both these foods contain large amounts of probiotic bacteria. Strains of L. plantarum are involved in the final stages of fermentation in both kimchi and sauerkraut, and they typically reach populations of more than 108 bacteria/mL by the end stages of fermentation,64–67 and thus are present in sufficient quantities to have therapeutic effects when consumed. Additionally, research found that many of the L. plantarum strains isolated from fermented foods can survive exposure to gastric acid and bile salts, thereby indicating an ability to survive transit through the upper GIT. These same strains were also able to adhere to intestinal epithelial cells, thus fulfilling three of the main criteria needed by desirable probiotic organisms.68 Kimchi and sauerkraut can be used as therapeutic tools in a similar manner to probiotic supplements and yogurts. However, the characteristics of the bacterial strains found in these fermented foods are not known, so therapeutic effects will not be as certain.
Some strains of L. plantarum isolated from fermented foods also utilize a mannose-specific mechanism to adhere to human intestinal cells. Many pathogenic bacteria and parasites (e.g., enterotoxigenic E. coli, Shigella spp., Vibrio cholerae, Salmonella spp., and Giardia lamblia) also utilize a mannose-specific binding mechanism.69,70 Hence, strains of L. plantarum compete directly with these microorganisms for a limited number of binding sites along the human GIT. The consumption of traditionally prepared kimchi and sauerkraut may thus play a role in the prevention and treatment of gastroenteritis caused by these pathogens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree