CHAPTER 41 Primary Tumors
INTRODUCTION
Primary tumors of the spine are uncommon, accounting for 5–10% of all primary skeletal tumors. At the Rizzoli Institute, 323 patients (adults and children) with primary spine neoplasms were seen over the past 50 years; 27% were malignant and 73% were benign.1 The most commonly encountered benign tumors of the mobile spine (the cervical, thoracic, and lumbar segments) include osteoblastomas, osteochondromas, giant cell tumors, hemangiomas, osteoid osteomas, and aneurysmal bone cysts. Common malignant primary tumors of the mobile spine include chordomas, chondrosarcomas, osteosarcomas, and Ewing’s sarcoma (Table 41.1). Less common malignant lesions of the mobile spine include solitary myeloma, solitary lymphoma, fibrosarcoma, and hemangioendothelioma.2 Chordomas and Ewing’s sarcomas are much more common in the sacrum. In fact, the incidences and clinical characteristics of many sacral tumors differ from those found in the mobile spine. Therefore, statistics and characteristics regarding sacral lesions are included in the discussion of these particular tumor types that are commonly found in the sacrum.
Table 41.1 Most Common Primary Tumors of the Mobile Spine
| Common Benign Primary | Common Malignant Primary |
|---|---|
| Tumors of the Mobile Spine2,5 (%) | Tumors of the Mobile Spine2 (%) |
| Osteoblastoma (20–23) | Chordoma (33) |
| Osteochondroma (13–23) | Chondrosarcoma (25) |
| Giant cell tumors (16–22) | Osteosarcoma (19) |
| Hemangioma (10–20) | Ewing’s (8) |
| Osteoid osteoma (7–21) | |
| Aneurysmal bone cyst (13) |
There is a strong correlation between patient age and likelihood of malignancy in primary tumors of the spine.3,4,5 In children, nearly 70% of primary tumors of the spine are benign,6 and 70% of primary spine tumors in adults (>21 years old) are malignant.7 As in many tumors, certain tumors of the spine have a predilection for certain age groups. In patients younger than 10 years, neuroblastoma, eosinophilic granuloma, and Ewing’s sarcoma dominate. Aneurysmal bone cysts, giant cell tumors, osteoid osteomas, osteoblastomas, and eosinophilic granulomas are the most frequent primary spine tumors found in adults less than 30 years of age. Patients between 30 and 50 years of age more often have chondrosarcoma, chordoma, lymphoma, and hemangioma as well as metastatic lesions. Those over 50 years of age mostly likely have metastatic disease, but may present with solitary myeloma or chondrosarcoma.8
The spine can be divided into anterior elements and posterior elements (Fig. 42.1). Anterior elements consist of the vertebral bodies, and the posterior elements include the remainder of the vertebra (pedicles, transverse processes, laminae, and the spinous process). Most malignant tumors, both primary and metastatic, occur in the anterior elements. Primary spine tumors located in the anterior elements have a 76% probability of being malignant. Those tumors located in the posterior elements are more likely benign (64%).6 Metastatic lesions of the spine are found in the anterior elements approximately 95% of the time. Common primary spine tumors involving the posterior elements are aneurysmal bone cysts, osteoblastomas, and osteoid osteomas. Primary spine tumors that have a predilection for the vertebral body include osteosarcomas, chordomas, solitary lymphomas, eosinophilic granulomas, giant cell tumors, and hemangiomas.8
CLINICAL PRESENTATION
As mentioned previously, early diagnosis is critical in primary spinal tumors. Early detection can lead to optimal local control and prevention of distant spread. Therefore, a high index of clinical suspicion and an awareness of symptoms is vital. A careful history describing the characteristics and timeline of symptoms is important in diagnosis and may direct the work-up and treatment. A proper history includes a summary of prior treatment and provides a baseline to evaluate the course of the disease and the effect of therapy.
Pain
Back pain is the most common presenting symptom in both primary (84%)5 and secondary tumors (>90%)9,10 of the spine. Tumor pain is typically unrelenting, progressive, and often present during the night, although many types of pain can present. These include local, axial, radicular, and myelopathic pain, which are discussed in detail in the following chapter. Pain at night is particularly suggestive of tumors such as osteoid osteoma and sometimes more aggressive lesions. Tumor pain may be localized to a specific spinal segment or reproduced by pressure/percussion over the involved area. Tumor expansion may erode the cortical margins leading to pathologic fractures. Both pathologic fractures and tumor growth may involve the spinal canal and neural foramina, with compression of the cord and/or nerve roots resulting in neurologic deficits as well as pain. Ongoing destruction may also lead to development of spinal instability.
A history of persistent back pain should be taken seriously and usually warrants further investigation. Patients with a history of irradiation or Paget’s disease of the spine warrant added vigilance due to their risk of secondary osteosarcoma.11 In general, pain from tumors commonly mimics the pain produced by nontumorous disorders. Thus, it is necessary for the clinician to have a high index of suspicion when dealing with back pain, even if the patient does not present with the characteristic types of pain associated with spinal tumors.
Neurologic impairments
Neurological presentation entirely depends on the level of the lesion and the degree of nerve or cord compression. Neurological manifestations, including radiculopathy and myelopathy, may present some time after the onset of pain. Forty-one percent of patients with primary spine tumors complained of a subjective sense of weakness on initial presentation.5 Malignant tumors are associated with a higher incidence of neurologic deficit than are benign lesions.12 Benign tumors of the spine can often present with a history of symptoms of long duration or may remain asymptomatic for some time.
Other symptoms
Systemic or constitutional symptoms tend to be more common with malignant or metastatic disease than in benign lesions. As such, constitutional symptoms such as fatigue, fever, and unexpected weight loss must be included in a careful review of symptoms when a malignant or metastatic lesion is suspected. Red flags that suggest malignancy of the spine are presented in Table 41.2.
Table 41.2 Elements of the Presentation that are Worrisome for Spinal Malignancy
| Red Flags |
|---|
| History of prior malignancy |
| Back pain worse at night/pain and wakes patient from sleep |
| Consistent progression of pain |
| Pain unchanged during rest or activity |
| Acute neurologic deterioration |
| Presence of a mass |
| Presence of constitutional symptoms |
PHYSICAL EXAM
Musculoskeletal inspection of the spine
The patient should be inspected for any obvious deformities of the spine and abnormal posturing. A bony prominence, kyphotic deformity, or acute angular scoliosis can be observed after vertebral collapse.13 Scoliosis is often associated with osteoid osteoma and osteoblastoma located on the concave side of the apical portion of the deformity. Spine tumors are rarely palpable due to the amount of tissue and muscle layers superficial to the spine. However, the spinous processes of the spine should be palpated, while paying special attention to any tenderness, masses, vertebral defects, and spastic paraspinal musculature. Range of motion testing in flexion, extension, rotation, and lateral bending should be carefully performed.
Neurologic examination
In evaluating neurologic function, motor, sensory, and reflex function must be assessed and recorded. Although weakness is rarely the first symptom of a spinal column tumor, it can be objectively identified in 55% of patients with malignant lesions and in 35% of patients with benign lesions.5 Sensory findings are less common than motor findings in patients with either radiculopathy or cord compression. However, testing with pinprick and light touch, particularly in the sacral dermatomes should be performed. Saddle sensory loss may be associated with tumors in the area of the cauda equina. Compression above the cauda equina often spares the sensation to these sacral dermatomes.14
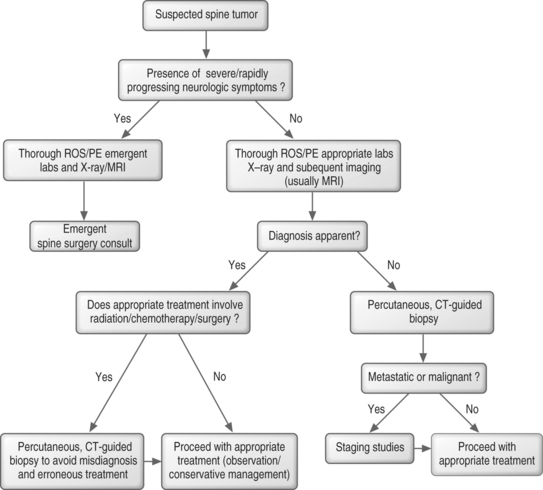
WORK-UP
Laboratory studies
The laboratory work-up in a patient with a suspected tumor of the spine can be involved. A complete blood count (CBC) with a differential is important when working up any suspected malignancy. Elevated erythrocyte sedimentation rates (ESR) and C-reactive protein (CRP) levels signal that an inflammatory process is involved, but cannot consistently differentiate an infectious process from a malignancy. Lactate dehydrogenase (LDH) levels can be elevated in sarcomas, and LDH isoenzymes 2 and 3 can suggest a diagnosis of lymphoma.15 To evaluate for liver cancer, alpha fetoprotein (AFP) levels are often obtained in patients with hepatitis C or those who are heavy drinkers. Carcinoembryonic antigen (CEA) is a marker of adenocarcinomas such as colonic, rectal, pancreatic, gastric, and breast.16 Prostate specific antigen (PSA) levels can help diagnose prostate cancer. A thyroid panel can help eliminate the suspicion of a rare thyroid primary, and parathyroid hormone (PTH) can be ordered to look for hyperparathyroidism. An elevated PTH level may lead to diagnosis of a brown tumor of the spine. The diagnosis of myeloma can be confirmed by the identification of monoclonal proteins in the serum or urine via serum protein electrophoresis (SPEP) or urine protein electrophoresis (UPEP);17 however, monoclonal proteins are more often absent or undetectable in solitary myeloma compared to multiply myeloma.18 A chemistry panel can be used to assess kidney function and allows calcium and phosphate levels to be followed to detect and avoid the development of malignant hypercalcemia. An elevated alkaline phosphatase level can also suggest a neoplastic bone disease.
Imaging techniques
Bone scan
Bone scanning (skeletal scintigraphy) utilizes a disphosphonate compound, tagged with technetium 99m, which becomes incorporated into bone by osteoblastic activity after intravenous injection. Bone scans may be instrumental in detecting tumors of the spine, and images of the entire body can be obtained in a fairly short period of time. One weakness is low specificity. Bone scans are known to be highly sensitive in localizing osteoid osteomas.19 This provides an earlier diagnosis and accurate localization of the tumor. Bone scans are also useful in identifying multifocal lesions and for evaluating metastatic disease.
Computed tomography
Computed tomography provides the best images of bone architecture and readily detects small areas of bone destruction or blastic change, although magnetic resonance imaging is more effective in detecting lesions before changes in bone structure can be demonstrated. In the past, CT was not considered a good screening tool for lesions in the spine, but with multidetector scanners, the entire spine can be scanned in great detail in under 5 minutes. Images can be reconstructed into any plane for the evaluation of bone alignment and extent of compression in a compression fracture. CT imaging can also provide the spine surgeon with an image of remaining bone in an abnormal vertebra, a factor in the feasibility of fixation. CT imaging is also valuable for planning and guiding percutaneous biopsies of vertebral lesions. CT imaging of the spine is especially useful in those patients who cannot undergo MRI (claustrophobic, cannot lie flat for long periods of time, or have implanted, metallic devices).
Myelography (conventional and CT-myelography)
To perform a myelogram, iodinated contrast material is instilled into the dural sac in order to detect external compression of the sac or space-occupying lesions within the spinal cord. Therefore, it is an invasive procedure with inherent risks. Before MRI, conventional myelography was the gold standard for detection of cord compression and intrinsic cord lesions, but it has been largely replaced by MR scanning, and by CT-myelography when MRI is contraindicated. Myelography may fail to reveal secondary sites of epidural spinal cord compression and has been shown to be less sensitive in diagnosing spinal tumors than MRI.20 CT-myelography, like conventional myelography, involves the instillation of contrast into the dural sac, but the amount of contrast used is much less due to the enhanced ability of CT to depict subtle contrast differences. By employing various window settings for the images, details of the paraspinal structures, bone, and dural sac contents are well demonstrated. Both conventional and CT-myelography may be used when metallic fixation devices have been placed in and around the spine and MRI is unable to provide adequate images. This, however, is becoming less frequent with the increased use of titanium spinal hardware.
Positron emission tomography
The most common radiotracer used in clinical positron emission tomography (PET) imaging is fluorine-18-fluoro-2-D-deoxyglucose (18F-FDG), which accumulates in areas of high glycolysis and membrane transport of glucose, both known to be increased in malignant tissue. Unlike the agent used in bone scanning, 18F-FDG may detect bone marrow-occupying lesions before cortical involvement occurs, thus detecting bone metastases before they can be found on bone scans. Sclerotic metastases, however, as found in some breast and prostate cancers, are less likely to be detected by PET as these lesions have lower glycolytic rates and are less cellular than lytic metastases.21 18F-FDG is not specific for tumors and may accumulate at sites of infection but is less likely to be detected at sites of degenerative change than technetium 99m, the agent used in bone scans. Therefore, it is somewhat more specific for tumors. PET also demonstrates metastases in soft tissue throughout the body, resulting in additional diagnostic value.
In addition to detecting spine tumors, PET may also be useful in distinguishing malignant lesions from benign. One study of 29 patients with cancer and spine abnormalities showed that two nuclear medicine physicians were in agreement in calling abnormalities benign, equivocal, or metastasis in 90%.22 In addition, 100% of abnormalities interpreted as benign or malignant were correctly identified. The only discrepancies were in three abnormalities that were interpreted as equivocal and which turned out to be metastatic. CT and/or MRI were important in arriving at the final diagnosis in equivocal cases.
The ability of PET to evaluate the response of bone tumors to chemotherapy has also been studied. In one study of patients who underwent preoperative chemotherapy for osteosarcoma, changes in tumor 18F-FDG uptake were correlated with percentage tumor necrosis on histopathology. Tumor necrosis was accurately predicted on PET scan in 15 out of 16 patients by visual assessment and in 14 out of 15 patients by final tumor to background ratio (TBR).23
Biopsy
Types of biopsy
There are two types of biopsy commonly used for biopsy of spinal lesions: percutaneous, guided and open, surgical biopsy. Both fluoroscopic-guided and CT-guided percutaneous biopsies can be utilized, and both are effective. The tip accuracy of CT makes it superior when dealing with small, deep-seated lesions especially in the cervical and thoracic regions.24 CT better allows selection of the optimal location to sample tissue. For lesions visible via fluoroscopic monitoring, fluoroscopic-guided biopsy offers real-time positioning of the needle. Open biopsy maximizes tissue retrieval and providing the highest diagnostic success rate; however, it is typically reserved for failed percutaneous biopsies due to the increased morbidity of the open procedure and greater risk of wound contamination with tumor. Regardless of which method is used, the goal is to obtain an adequate amount of tissue while minimizing complications.
Biopsy success rate
Accurate diagnosis of tumorous and nontumorous lesions using CT-guided biopsy is achieved more than 90% of the time.24–26 In lesions with central necrosis, the ability to obtain the correct diagnosis may be enhanced by obtaining tissue from the periphery of the lesion. In paucicellular aspirates, a cell block can be prepared or additional tissue, such as a core biopsy, can be obtained. If histology yields only peripheral blood in an obviously destructive mass, biopsy can be repeated, by directing the needle/device at a slightly different area of the lesion.26 If indicated, corticosteroids should ideally be administered after biopsy due to their lytic effect on certain tumors, including leukemia; this lytic effect can lead to a nondiagnostic biopsy.
Percutaneous biopsy of solitary lesions
The approach to the percutaneous biopsy of a solitary spinal lesion is fairly straightforward. Usually, the approach involves the shortest path to the lesion that does not place vital structures at risk. For biopsies of the spine, this typically involves a posterior approach; however, in the cervical spine anterolateral approaches are often used. A posterior transpedicular approach is often used to biopsy lesions in the vertebral body. The transpedicular approach helps to avoid vital structures while minimizing the amount of tissue susceptible to tumor contamination of the needle tract. Virtually any lesion within the vertebral body of cervical, thoracic, or lumbar vertebrae can by accessed via this approach.34 Lesions located in the posterior elements are typically easy to biopsy with a direct approach. Occasionally, primary lesions of the spine can locally metastasize to other vertebrae. In this instance, the decision of which lesion to biopsy is important and should be based on several factors including the size of the lesion, radiologic morphology, location along the spinal column, and location within the vertebra. These factors are discussed further in the next chapter.
Complications of biopsy
Biopsies of potentially tumorous lesions should be well planned. It is well known that inadequate or inappropriate biopsies adversely affect outcome. Complications arising in these unsound biopsies include disability due to more complex resection, loss of function, local recurrence, and death.28 The surgeon that will be performing the definitive surgical procedure, if further surgery becomes necessary, should always perform the open biopsy. This ensures that the subsequent surgery can be performed using the optimal incision and approach, while excising the biopsy incision and tract. This can also help eliminate unnecessary and improperly performed open biopsies.
Complications of percutaneous needle biopsy include bleeding, infection, neurologic compromise, fracture, biopsy tract contamination, and death, although serious complications are rare. Due to the risk of tumor contamination of the biopsy tract,29 the needle tract should be excised if a subsequent surgery is indicated, although this is somewhat controversial. Whenever possible, guided biopsies should be done at the same institution where definitive surgical treatment will occur. Typically, pathologists at the larger referral centers will be more experienced with uncommon primary and secondary malignant tissues obtained from the spine and will typically review specimens despite previous histologic diagnosis from outside institutions. Also, a team approach between the interventional radiologist and the treating surgeon is more likely to produce a favorable result.
BENIGN TUMORS OF THE SPINE
Eosinophilic granuloma
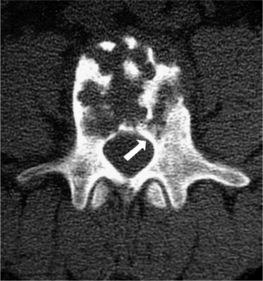
Also known as Langerhans cell histiocytosis (LCH), eosinophilic granuloma is a benign and self-limiting process that can lead to focal destruction of bone. It is most prevalent in children, with half of patients under the age of 10 years. The etiology is unknown and the lesion is comprised of lipid-containing histiocytes from the reticuloendothelial system and eosinophils. Lesions are most common in the skull although virtually any bone may be affected, with vertebral involvement occurring in approximately 10–15% of cases. The most common appearance is a well-circumscribed, punched-out lesion with no periosteal reaction. Less common is the moth-eaten pattern with periosteal reaction. Both are demonstrated in Figure 41.2. Vertebral destruction with complete collapse of the vertebral body can occur and is classically referred to as ‘vertebra plana.’ Multiple vertebrae may occasionally be involved. Collapse can produce pain and spasm of the paraspinal muscles. Deformities in the form of gibbus or kyphus may develop in some cases. Diagnosis is confirmed by needle or open biopsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree