Chapter 32 Prevention and Management of Chronic Wounds
The financial burden of chronic wound care is immense and will continue to increase as the population increases, particularly in industrialized countries. In the United States alone the total annual direct cost of chronic wound care (including professional expenses, hospital costs, and complications) is estimated to be $20 to 25 billion.35,75,98 Four ulcer types account for most of this economic burden: pressure ulcers ($5 to 8.5 billion), ischemic and neuropathic ulcers ($7 billion), and venous ulcers ($1.9 to 2.5 billion).72,168 Because prevention and aggressive treatment of early ulcers reduces costs, there is an economic impetus for the growth of outpatient wound centers. During the past two decades, the number of wound care treatment centers has increased from very few to more than 900.5
Another trend in wound care derives from the current revolution in molecular biology, gene therapy, biomaterials, bioengineering, tissue engineering, and stem cell research. Stem cells are now being applied to chronic wounds in animal model research and in human trials as well. Chronic wound products derived from recombinant DNA technology have been in place since the late 1990s.85 Dramatic changes in wound care will continue to occur as gene therapy involving growth factors undergoes human trials. A diversity of synthetic and natural skin grafts is now commercially available for various wound types.181 Physiatrists can potentially use all these interventions in the care of patients in the postacute setting.
Scope of the Problem
Definitions
The National Pressure Ulcer Advisory Panel (NPUAP) defines a pressure ulcer as localized injury to the skin and/or underlying tissue, usually over a bony prominence as a result of pressure or pressure combined with shear and/or friction.7 Pressure ulcers are associated with immobility, impaired mental status or sensation, poor hygiene or nutrition, dark skin color (associated with difficulty in detecting early-stage ulcers), and multiple comorbidity factors. High standards of nursing and medical management are a key to prevention of ulcers in immobilized patients.
Neuropathic ulcers result from repetitive trauma to hyposensate distal extremities (e.g., feet), usually on weight-bearing bony prominences such as metatarsal heads. In the case of uncomplicated neuropathic ulcers, the circulation is usually functionally intact.95 A cornerstone for this treatment is mitigating abnormal repetitive pressure and shear stress and addressing the causative and treatment factors associated with the underlying neuropathy.
Epidemiology of Chronic Wounds
Pressure Ulcers
A national survey of 116 acute care facilities involving 17,560 patients reported an incidence for pressure ulcers of 7% and a prevalence of 15%. This survey also indicated that 90% of the ulcers were at stage I or II, and 73% occurred in patients older than 65 years.206 Patients in critical care units could run a higher risk for developing pressure ulcers, as evidenced by an incidence range from 38% to 124% in a recent study.179
Long-term facilities are frequently the disposition for patients with pressure ulcers sustained during acute hospitalization. In one large study involving 92 nursing homes and 2343 patients, prevalence rates for all stages of pressure ulcers were 8.52%, and those in stage II or greater were 5.31%.44 A “do not resuscitate” (DNR) order confers a significant risk factor for pressure ulcer formation.162 It is notable that most pressure ulcers heal within 1 year.28
The wide variation in the prevalence of pressure ulcers in long-term care facilities of 2.6% to 24%28 can be partially explained by the variable implementation of policies and procedures related to incontinence, inspection, turning, positioning, range of motion, and nutrition. Because of this wide disparity, the prevalence of pressure ulcers is seen or accepted as an important index of care quality and is monitored by federal and state governments in the Minimum Data Set (MDS). It is now becoming recognized, however, that some pressure ulcers might be inevitable, occurring near death in what has been termed “skin failure.”210
Individuals with spinal cord injury (SCI) and associated comorbidity are at increased risk for the formation of pressure ulcers. These patients are paralyzed below a certain level of the body, which limits their ability to relieve the pressure acting on the immobile portions of the body. The sensory loss that results from SCI also renders the patients unaware of the impending or existing injury caused by prolonged pressure. Another factor is that the neural and metabolic regulatory mechanisms for the maintenance of adequate tissue blood flow are impaired in patients with SCI.118,164 The overall consequence is an incidence of such ulcers ranging from 23% to 33% or more per year and a lifetime prevalence of up to 95% in these individuals.76,78,192
Another group at risk for pressure ulceration is the elderly population. The prevalence of pressure ulcers among those 65 years of age and older varies from 0.31% to 0.70% and is even higher in individuals 85 years and older.125
The incidence and prevalence of pressure ulcers in the pediatric population are lower than in adults.12 Pressure ulcers can still pose significant complications in children, however, particularly in the setting of severe illnesses and in those with neurologic impairments consequent to cerebral palsy, spina bifida, and traumatic and acquired SCI.12,145,215
Diabetic, Ischemic, and Neuropathic Ulcers
Neuropathy, arteriosclerosis, and microvascular disease are part of the natural history of diabetes. These comorbidities create a high risk for development of ischemic or neuropathic ulcers in individuals with diabetes, as evidenced by a 12% to 25% lifetime risk for developing a foot ulcer.185 Diabetic foot ulcers in turn increase the likelihood of lower limb amputations. Hospitalization rates for lower limb amputation are 13 times higher for persons with diabetes than for the general Medicare population.39 The age-adjusted nontraumatic lower limb amputation rate in 2005 was 3.9 per 1000 persons with diabetes.38 The first major limb amputation for those with diabetes is often followed by a second major amputation, because 28% to 51% have a contralateral amputation within 5 years.167 There were approximately 71,000 U.S. hospital discharges for nontraumatic lower limb amputations in persons with diabetes in 2005.40
The cost of a single major amputation is conservatively estimated at $100,000, including the acute hospital stay, surgeries, rehabilitation, and prostheses.212 Multiplying the cost per amputation and number of amputations among those with diabetes from the latest Centers for Disease Control and Prevention (CDC) data (2005), the cost burden is $7 billion. In the 1990s the U.S. Public Health Service called for a 40% reduction in diabetic-related amputations by the year 2000 and urged prevention and earlier treatment to achieve that goal.6 Unfortunately the CDC data indicated that there was actually a 42% increase in hospital discharges for nontraumatic lower limb amputations in persons with diabetes between 1990 and 2005.29 These disappointing trends suggest that preventive and early intervention strategies elaborated in this chapter need to be more systematically implemented.
Venous Stasis Ulcer
Approximately 1% of the U.S. population and 3% of the population aged over 65 have venous stasis ulcers, with the incidence rate twice as high for women compared with men.124 An estimated 3 million Americans now suffer from venous ulcers. Because only 600,000 per year are treated, the majority of chronic venous ulcers go untreated. The cost of treatment per patient runs at a median of $3036 per year.149 Of venous ulcers, 20% to 30% are intractable, are present for more than 1 year, and have a surface area exceeding 10 cm2. Intractable venous ulcers incur higher costs, approaching $10,000 per case.148 At an average cost of $3000 per ulcer, the total direct cost is estimated at $2 billion (which does not include the hidden costs of untreated ulcers). Venous ulcers also cause an estimated 2 million lost workdays.155
Wound Physiology and Pathophysiology
Definitions
The Wound Healing Society defines healing as complete closing of the integument.117 Skin wounds that heal by primary intention are similar to incisions that are created surgically and then heal rapidly and without complication in 3 to 14 days. More complicated are the wounds that heal by secondary intention. Secondary intention wounds are large tissue defects that fill by granulation followed by epithelialization. Wound closure occurs to some extent because of wound contraction.
Process of Normal Healing
The major phases of wound healing are inflammation, proliferation/provisional matrix formation, repair, and remodeling.85 After tissue injury, the body reacts to impede the blood loss. Platelets that come in touch with the injured vascular surface form platelet plugs and release chemical stimuli, including several growth factors. At the same time a complex cascade of procoagulant molecule activation culminates in the conversion of fibrinogen to fibrin, which creates a fibrin mesh that forms the scaffolding of blood clot that further traps platelets that increase growth factor stimulation. Inflammation is characterized by breakdown of the preexisting tissue scaffolding and cleanup of extracellular and pathogen debris. Matrix breakdown also enables migration of neutrophils, macrophages, epidermal cells, and fibroblasts into the site of injury, enabling its ultimate repair. Repair is analogous to the construction of a new building, with a framework of extracellular matrix components (a provisional matrix of glycosaminoglycans, which are protein–sugar complexes and fibronectin) attached to “rivets” (cell attachment sites called integrins). On this framework are laid reinforced “girders” of type I collagen, which are secreted in sections (fibrils) and self-assemble extracellularly. Over this structure (while under construction), a “roof” of epidermal cells advances over the defect to provide a durable cover, and a “plumbing” network of neovessels begins to form to supply oxygen and nutrients (i.e., neoangiogenesis). Remodeling of the dermal matrix occurs after closure, during which collagen fibers are preferentially retained along lines of stress.
Biochemistry of Normal Healing
Wound healing is a complex but orderly process involving initial inflammation, proliferation of parenchymal and connective tissue cells, and remodeling.49 As noted earlier, after tissue injury the body reacts to impede the blood loss. Platelets that come in contact with the injured vascular surface form platelet plugs and release chemical stimuli such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor–β (TGF-β). In a parallel process, a complex cascade of procoagulant molecule activation culminates in the conversion of fibrinogen to fibrin, creating a fibrin mesh that forms the scaffolding of blood clot.49
Growth factors such as TGF-β, PDGF, fibroblast growth factor (FGF), and cytokines (including interleukin-1 [IL-1] and tumor necrosis factor–α [TNF-α]) stimulate the fibroblasts to migrate to the injury site, proliferate, and secrete matrix metalloproteinases (MMPs). The released MMPs aid in the breakdown of extracellular matrix (ECM). Degradation of ECM results in the release and activation of growth factors such as TGF-β1, PDGF, basic fibroblast growth factor (bFGF), and interferon-γ (IFN-γ).49
Various immune cells including neutrophils, monocytes, macrophages, mast cells, and lymphocytes are recruited to the wound site by growth factors derived from platelets and ECM. These cells clear tissue debris, bacteria, and foreign materials from the wound site by phagocytosis and synthesize various growth factors and cytokines. Neovascularization also is promoted by VEGF.49
Activated fibroblasts further synthesize and deposit major ECM components such as collagen and fibronectin that impart strength to the healing wound. Collagen is secreted into the ECM in the form of tropocollagen. Three strands of tropocollagen then polymerize to form collagen.49
A portion of fibroblasts are converted to myofibroblasts under the combined influence of mechanical tension, TGF-β, and cellular fibronectin extra domain type A (ED-A).77 Myofibroblasts aid in shrinking and closing the wound by using an actin–myosin contractile system involving α–smooth muscle actin. These cells also synthesize collagen types I and III. Myofibroblasts disappear after epithelialization via apoptosis (preprogrammed cell death).104
In parallel with the changes in the wound stroma, the epithelial cells around the wound margin migrate over the defect to provide a durable cover of the wound. The epithelial cells undergo a series of phenotypic changes termed epithelial–mesenchymal transition. In this transition epithelial cells assume a fibroblast-like appearance and gain motility and invasiveness. E-cadherin molecules facilitate epithelial adhesion to the basement membrane and to adjacent epithelial cells; correspondingly adhesion is suppressed by suppressed, N-cadherin molecules found in the mesenchymal cells.204
The stimuli that trigger the migration of epithelial cells include gradients of chemoattractants, pressure from adjacent cells, and endogenous microelectric fields.218 Basement membrane components are deposited as the cells move. After the migrating fibroblast-like cells have covered the wound site, they revert to epithelial cells through a process known as mesenchymal–epithelial transition.204
Pathophysiology of Chronic Wounds
Pathomechanics
Pathomechanics implies noxious application of pressure on and shear stress tangential to the skin surface. Unrelieved pressure is the critical factor in development of pressure ulcers. Prolonged pressure leads to ulcers if it exceeds the tissue capillary pressure of 32 mm Hg. Known as the critical tissue interface pressure, this benchmark is 75 years old and serves as a basis for design of clinical pressure-relieving surfaces.174
The critical interface pressure is the pressure above which a tissue cannot be loaded for an indefinite period without resulting ulceration. An inverse, hyperbolic relationship exists between pressure and the duration of pressure application necessary to cause ulcers. Unrelieved pressure 4 to 6 times the systolic blood pressure causes necrosis in less than 1 hour. Pressure below systolic blood pressure, however, might require 12 hours to produce a similar lesion. This hyperbolic relationship has been confirmed for several animal models115 and can serve as the basis for turning patients every 2 hours.169
The surface pressure, however, might not be a good measure of the true pressure in deep tissues.26 Deep muscle layers that cover bony prominences are often exposed to higher stresses than overlying skin surfaces. Prolonged compression increases muscle stiffness around the bone–muscle interface, which further stresses the muscles. This makes the muscle even more prone to ischemia and infarction. The injury progresses from deep to superficial tissues in an inverted cone shape. As a result, when we see the injury on the skin, it is literally “the tip of the iceberg.”82
Shear stresses exacerbate the tendency for ulceration caused by pressure by lowering the ulceration threshold sixfold.57 The classic example of shear stress generation is when a patient reclines in a hospital bed with the head of the bed elevated—the human–machine interface. This position places the sacrum at increased risk for tissue breakdown. Once ulcers form, they are empirically more sensitive to pressure and shear stress than intact skin.
Recent evidence from cell and tissue culture studies suggest that sustained cellular deformation can cause tissue death primarily as a result of apoptosis even under normal supply of nutrients and oxygen.24,25,202 These results indicate that pure compression without any metabolic derangements associated with hypoxia/ischemia might be an etiologic factor leading to tissue damage. In particular, this mechanism of injury is implicated in the formation of deep tissue injuries.81
Chronic Hypoxia
Chronic hypoxia results from poor inflow of blood typically as a result of arteriosclerotic narrowing proximal to hypoxic skin. Chronic ischemia blunts granulation tissue deposition, proliferation of fibroblasts, mononuclear cell infiltration, delayed epithelialization, and probability of wound closure.152,213,214 On the subcellular level, α1(I) procollagen is down-regulated, and TGF-β signaling in fibroblasts is altered (e.g., lower TGF-β1 mRNA levels and down-regulated TGF-β type II receptors) under the condition of chronic hypoxia.114,182 These changes associated with chronic hypoxia contrast with the stimulatory effect of acute hypoxia on wound healing.193
Reperfusion Injury
The current practice for managing pressure ulcers is turning the patient every 2 hours.160 This practice invokes a hypothesis that repositioning the patient every 2 hours could be too infrequent and could result in cycles of alternating ischemia and reperfusion in loaded tissues, thereby increasing the risk of ischemia–reperfusion injury. The question still remains about the appropriate turning frequency for patients at risk because of an inadequate evidence base for turning schedules.
Ischemia–reperfusion injury involves reactive oxygen species that overwhelm endogenous antioxidants, resulting in a cascade of events including mast cell degranulation, recruitment of neutrophils to the endothelial wall, arteriolar constriction that limits tissue perfusion, and increased vascular permeability that leads to inflammation and edema.94,173
Animal studies have shown that in young animals, chemotaxis, oxidant release, and phagocytosis by neutrophils play key roles in tissue damage after reperfusion. In older animals, increases in oxidative stress and mast cell density and action appear to have a more significant perturbation on the antioxidant defense.94,173
Patients with diabetes mellitus are at increased risk of reperfusion injury because there are decreased levels of microvessel nitric oxide (which is a potent vasodilator that protects the vascular endothelium from reperfusion injury).200
Edema, Impaired Oxygen, and Nutrient Exchange
Edema is one of the major factors associated with the pathogenesis and maintenance of chronic wounds. Venous ulcers extravasate fibrinogen and fluid across the microvasculature endothelium due to venous congestion and back pressure, leading to excess protein-rich interstitial fluid. The excess osmotic pressure of interstitial fluid is thought to sequester growth factors, which are then unavailable to heal the edematous skin.68 Venous congestion can also lead to endothelial damage, causing neutrophils to marginate and release free radicals and collagenases. This process further increases membrane permeability to macromolecules, osmotic pressure and fluid shifts, and worsening edema.66
Growth Factor Abnormalities
Chronic wounds contain elevated levels of a variety of proteases such as interstitial collagenases (MMP-1 and MMP-8), gelatinases (MMP-2 and MMP-9), and the stromelysins (MMP-3, MMP-10, and MMP-11). Chronic wounds also have various levels of serine proteases and elastases, all of which collectively degrade the ECM of the wound bed.217 Tissue inhibitor of metalloproteinase, plasminogen activator inhibitor, α1-protease inhibitor, α1-antichymotrypsin, and α2-macroglobin usually balance the activity of the MMPs but are overwhelmed in a chronic wound mainly by the influx of large numbers of neutrophils.
Chronic Inflammation
Although colonization with bacteria is normal and can even be helpful during the initial healing phase, critical colonization and local infection impede healing. Wounds that have greater than 105 organisms per gram of tissue tend not to heal98 and are “stuck” in the inflammatory stage. The process of decreasing bacterial bioburden through “wound bed preparation” is discussed below.
Comorbidities
The factors that contribute to chronic wound persistence are diverse, interactive, and cumulative, and they affect the whole person. Predisposing conditions include aging, SCI, and diabetes, among many others (Table 32-1). These comorbidities interact and are cumulative at many levels to contribute to pathomechanics, hypoxia, reperfusion injury, and local inflammation. Pressure ulcers in individuals with SCI are slow to heal. This slow healing process is thought to be caused by neural and metabolic derangements in the denervated skin such as a limited inflammatory response as a result of vascular impairments, decreased availability of oxygen, reduced synthesis of collagen molecules, and decreased fibronectin resulting in impaired fibroblast activity.161 Aging is also associated with slower wound healing and subsequent functional wound closure. Reduced proliferation of fibroblasts, keratinocytes, and vascular endothelial cells, decreased collagen synthesis, and diminished fibroblast response to growth factors are associated with advanced age.90,159,165
Table 32-1 Comorbidities Associated With Chronic Wounds
| Condition | Pathophysiologic Effect Related to Wound Healing |
|---|---|
| Spinal cord injury | Vasomotor instability (>T6 level), insensitivity, denervation atrophy, spasticity, contractures, bowel/bladder alterations78 |
| Elderly | Reduced skin elasticity and altered skin microcirculation,199 co-morbidities, reduced healing rate noted clinically and in animal models90 |
| Diabetes | Insensitivity, microangiopathy and altered inflammatory response,139 foot deformities (intrinsic minus, Charcot), blunted reactive hyperemia, reduced incision breaking strength,154 and contraction92 in animal models |
| Malnutrition | Negative nitrogen balance, cachexia, immunosuppression |
| Anemia | Local hypoxia |
| Arteriosclerosis | Local hypoxia |
| End-stage renal disease | Transient dialysis-related hypoperfusion, arteriosclerosis, microangiopathy |
| Steroid medications | Reduced healing rate in animal models, immunosuppression |
| Transplant recipients | Immunosuppression |
| Smoking | Hypoxia, vasoconstriction, increased blood viscosity |
| Parkinson disease | Immobility |
| Osteoporosis | Bony prominences |
| Upper motor neuron disease | Immobility, contractures, bowel/bladder alterations |
| Dementia | Immobility, malnutrition, contractures, bowel/bladder alterations |
| Acutely ill (intensive care unit related) | Hypotension, immobility, bowel/bladder alteration, malnutrition, increased metabolic demands |
| Noncompliance, abuse and neglect | Multifactorial |
Clinical Wound Assessment
Wound Area and Volume Assessment
Wound Area
The most straightforward technique is to document the wound’s perpendicular linear dimensions (typically in centimeters); the maximum distance is length, and perpendicular distance is width.2 Although linear dimensions can be performed rapidly, they have limited sensitivity to change in wound size, limited information about shape, and overestimate the wound area by up to 25%.88 Because of these concerns, serial wound outlines are often performed. Manual tracing, a useful and inexpensive technique, involves drawing the wound outline on clear plastic (e.g., acetate sheet for transparencies) with an indelible marker. Counting the number of squares inside the wound periphery on the acetate sheet gives an estimate of wound area.2 These drawings then become part of the patient’s permanent record. Inspection allows immediate appraisal of progress, and if the wound has increased in size, the clinician can modify the treatment without delay.
Wound Volume
For a first approximation of volume, area is multiplied by depth to find volume. The volume of a rectangular solid calculated in this manner overestimates the volume of pressure ulcers. Computing volume of a spheroid is more accurate.88 By either calculation, using depth is most accurate for deep cavities and least accurate for shallow, irregularly shaped wounds.
Wound Appearance
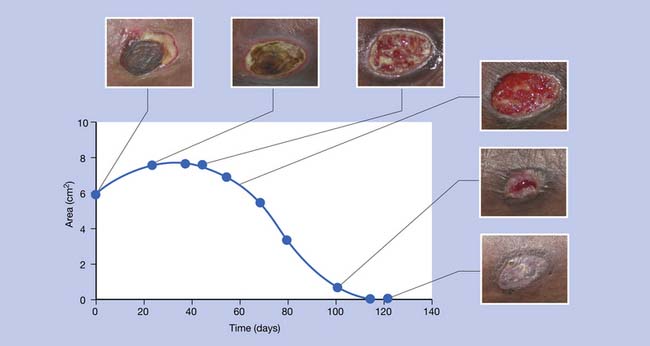
To describe wound appearance, the red–yellow–black system is often used (wounds classically transform from black, to yellow, to red as they heal; Figure 32-1). One method is to classify a wound as the least advantageous of the three colors that it displays.123 This method has been criticized as simplistic. The color of the wound surface can be alternatively described as a relative percentage of the three colors. Digital photography makes it convenient to serially classify with wound color.
Computerized Assessment of Wound Geometry
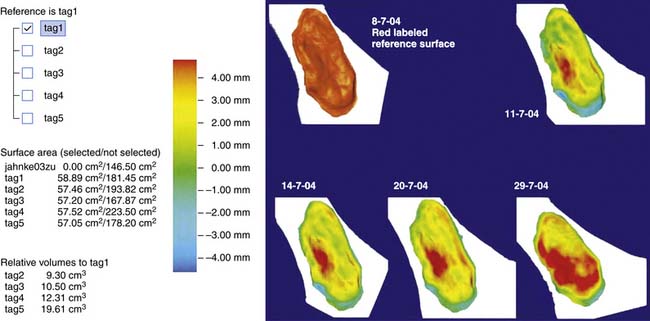
Computerized systems have been developed for more accurate and automated assessment of wound geometries. Digital planimetry enables automatic calculation of the wound area once the wound edge is traced manually or digitally.95 Excellent interrater and intrarater reliabilities and concurrent validity have been reported for computerized planimetry compared with other methods of wound area determination.83,116,216 Stereophotographic systems use multiple cameras to reconstruct the three-dimensional shape of the wound (Figure 32-2). This gives the most exhaustive representation of the geometry of the wound surface because virtually no loss of information is involved. To assess the volume and depth of the wound, the geometry of the original intact skin overlying the wound is required. The wound volume is the volume of the region enclosed by the recorded wound surface and the surface of the original intact skin. Because the original skin surface has already been destroyed, it is reconstructed computationally by interpolating the normal skin neighboring the wound. Regions of fast and slow healing (e.g., via granulation tissue induction) can also be tracked by following the geometry of the wound surface (Figure 32-3).
In summary, wound measurement techniques need to be accurate, reliable, and reproducible. When used for clinical and research purposes, any measurement technique must possess a high level of sensitivity and specificity. Measurement techniques must also be easy to use. Volumetric wound assessment is paramount for the refinement and advancement of modern wound measurement techniques., The current state-of-the-art stereographic volumetric systems, however, are not yet readily available to the clinician.
Perfusion Assessment
Macrocirculation
Ankle–Brachial Index and Pulse Volume Recording
Ankle–brachial index (ABI) is the ratio of systolic blood pressure of the ankle to that of the arm (brachium).88 Normal ABI is 0.8 to 1.3 and can be determined by a portable Doppler instrument and a blood pressure cuff. A series of cuffs can be used to obtain a pulse volume recording (PVR), which is continuous monitoring of pressure within cuffs applied to the thigh, calf, and ankle. These segmental pressure traces are checked for bilateral symmetry and waveform morphology. Pulse pressure curves of normal arteries resemble steep isosceles triangles. Flattened pulse pressure waveforms or asymmetric indices suggest proximal flow compromise from arteriosclerosis. For patients with diabetes, calcinosis of the tunica media often falsely elevates ABI. Because calcinosis is less likely to involve foot arteries, it is often helpful to determine toe pressures. To determine toe pressure, a tiny cuff fits over the hallux and a Doppler probe records systolic pressure. A pressure greater than 40 mm Hg is a good prognostic indicator of healing a foot ulcer. Toe pressure and ABI determination use a portable Doppler instrument and do not require a dedicated vascular laboratory.23
Angiography
Angiography typically involves injecting radiopaque dye into the proximal arterial tree. Conventional dye angiography is the gold standard for imaging macrovessels. It is an accurate prognosticator of limb salvage based on burden of arteriosclerosis visualized in the lower extremity.64 A noninvasive alternative to conventional angiography is magnetic resonance angiography, which requires no contrast dye and is superior to conventional angiography in visualizing arteries of the ankle and foot.129
Microcirculation
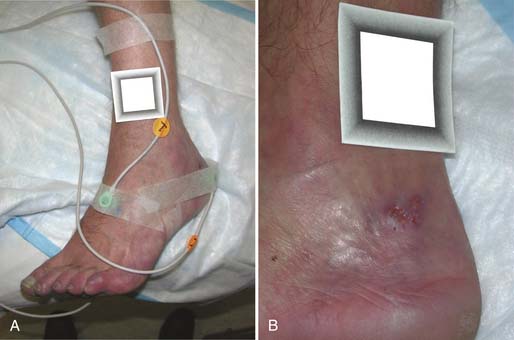
In the skin, blood flows through arterioles, capillaries, and venules in the papillary and reticular dermis.32 A direct and absolute measure of microcirculation is transcutaneous oxygen (TcPO2). TcPO2 is in essence a “blood gas” of the skin. The normal TcPO2 is greater than 50 mm Hg, with the ischemic level less than 20 mm Hg.152 A typical pattern of electrode placement is the upper leg, foot dorsum, and periwound (Figure 32-4).
TcPO2 prognosticates the healing rate of neuropathic and ischemic ulcers. Its predictive value is uniquely strong for patients with diabetes who typically have distal arterial calcinosis.152 For this population, TcPO2 is more accurate than toe pressures to prognosticate healing.110 The surgical literature reports that TcPO2 also prognosticates success of incisional healing at the transtibial level170 and foot level.156 A disadvantage of TcPO2 is that the technique gives values that are inaccurately low over areas of callus and transiently low in the setting of infection80 and in the immediate postoperative situation. TcPO2 measuring instruments are expensive ($2500 to $5000 per channel) and time-consuming (20 minutes to 1 hour to obtain a reading).
The most complete picture of lower extremity perfusion comes from combining TcPO22 and segmental pressures. For example, very low distal segmental pressures (i.e., ABI <0.4) but normal foot TcPO2 can indicate collateralization around an arterial blockage. Under these conditions, normal TcPO2 suggests good healing prognosis and a favorable chance of limb salvage despite the low ABI.88
Pressure and Shear Stress Assessment
Sensor arrays and point sensors are commercially available that can help to ensure that insoles and seating cushions distribute pressure over a maximum surface area.88 Two-dimensional sensor arrays (e.g., resistive, capacitive, and piezoresistive types) provide accuracy and good resolution of pressure distribution. Although pressure is well quantified, transducers for shear stress have yet to be perfected for routine clinical use.
Skin Biopsy
Although there are many biopsy types (e.g., excisional, incisional, and shave), the 3-mm punch technique is amenable for physiatric practice (low risk of bleeding and small skin defect). The method for biopsy involves selecting a site over soft tissue that is distant from subcutaneous arteries, fascia, or bone. The site selected should include periwound skin and a small sample of the wound base. Lidocaine without epinephrine (1 to 2 mL) is infused through intact periwound skin toward the wound center. The punch is inserted with a pressing, twisting motion, and a sample is harvested for histologic analysis.193
General Principles of Treatment
Wound Bed Preparation
Chronic wounds (unlike acute wounds) tend to be heavily colonized by bacteria. Colonization, however, does not equal infection.133 Wounds exist on a continuum between frank bacterial invasion, critical colonization, colonization, and healing. As wounds heal (and bacterial virulence decreases), wound appearance transforms from black, to yellow, to dull red, to bright red (see Figure 32-1). With beefy granulation tissue formation, there is a substantial decrease of drainage and pain, with management implications. The therapeutic processes for “coaxing” a chronic wound to granulate have been referred to as “wound bed preparation.”67 Once the wound bed is prepared, the goal is to maintain a moist environment. In a moist environment the epithelium advances and adjoins without having to digest eschar (i.e., “scab”), which optimizes the rate of healing.209 Wound fluid is also rich in growth factors that help promote closure.
Debridement
Sharps Debridement
Less aggressive outpatient or bedside sharps debridement is performed by professionals from many disciplines, including physiatrists, and has been recognized as a distinct debridement method in a recent clinical practice guideline.78 A series of sharps debridements is typically required to have the same effect as one surgical debridement. Sharps debridement is commonly performed in the outpatient setting as part of routine wound care with minimal blood loss. Any pain concerns are managed with topical analgesia. A local anesthetic, lidocaine 5%, or EMLA cream (lidocaine 2.5% and prilocaine 2.5%)34 can be applied 5 to 15 minutes before debridement. Premedication with a fast-acting oral nonopiate and/or oral opiate analgesic can also be helpful. Debridements should be performed at regular intervals to ward off infection because devitalized tissue supports the proliferation and growth of pathogens.
Pain is usually not a problem for patients with neuropathic ulcers. These ulcers develop callus or a pseudocapsule that is debrided in an inverted-cone pattern using a scalpel and forceps, which is referred to as “saucerization.” Debridements should be done periodically because neuropathic ulcers readily form calluses even with very little weight bearing, and a callus increases the mechanical tension in the wound.95 Venous ulcers do not develop calluses but frequently produce a yellow fibrinous slough over the wound bed (which can be removed with curettage). If a stasis-like leg ulcer expands after debridement, the diagnosis of pyoderma gangrenosum should be considered.162
Note that slough and necrosis can be similarly removed from pressure ulcers. Because pressure ulcers tend to be deeper than they are wide, knowledge of pelvic anatomy is essential, such as the location of the inferior gluteal artery. Particular concern is raised if the pressure ulcer is “unstageable” (e.g., when it has a 100% yellow surface). A completely nonviable surface raises concern that the pressure ulcer extends well into deep tissue planes. Such wounds should be sharp debrided with great care (because of the risk of bleeding), with a very low threshold for surgical referral.
Ischemic wounds also must be sharp debrided with great care because the blood supply might not be adequate to support a repair response (which can lead to further necrosis). If eschar appears to be contiguous with bone (e.g., black eschar over the calcaneus), that bone will likely not heal if exposed. In this circumstance, the eschar should be left dry unless infection supervenes. Where black eschar overlies soft tissue and reperfusion is possible, the authors advocate topical use of silver sulfadiazine, which has a broad antibacterial spectrum and softens eschar. This forms “pseudoeschar,”140 which can self-dislodge or is readily removed by careful periodic sharps debridement.86,87,133
Mechanical Debridement
Mechanical debridement is accomplished by whirlpool treatments, forceful irrigation, or use of wet-to-dry dressings. Wet-to-dry dressings involve placing unraveled, moist gauze into the lesion so that all sections of it are touching the dressing, then allowing the dressing to dry. When the dry dressing is subsequently removed, necrotic tissue is removed with it. Although wet-to-dry mechanical debridement is a ubiquitous method, it is increasingly criticized as inefficient and unnecessarily painful.151
Enzymatic Debridement
Several major types of enzymatic debridement agents are currently on the market. These include collagenase (e.g., Santyl) and papain-urea (e.g., Accuzyme and Gladase). Urea denatures protein, which increases the effectiveness of the nonspecific protease papain. Collagenase is said to be better for preparing the wound bed because it does not digest growth factors or matrix proteins.67 The use of enzymatic debriding agents can be the most cost-effective method for treating well-perfused partially necrotic pressure ulcers where sharps debridement is not readily available, such as in the skilled care setting.133 They are applied with daily change of dressings, until wounds are free of slough or eschar. It should be noted that enzymatic debridement might increase pain and drainage, requiring adjustments to dressing change schedules. The efficiency of enzymatic debriding agents is increased by “cross-hatching” the eschar with a scalpel.183
Autolytic Debridement
Autolytic debridement involves the use of natural proteases and collagenases in wound fluid to digest nonviable material. This method can be very effective when used under semiocclusive or occlusive dressings. Autolytic debridement is contraindicated, however, when wounds are infected or critically colonized. It should also be kept in mind that if slough or necrotic material is excessive, drainage can accelerate the maceration of periwound skin and increase wound size. As a rule of thumb, in a healing wound, the area of viable granulating base should be greater than 50% of the entire wound surface.133
Dressings
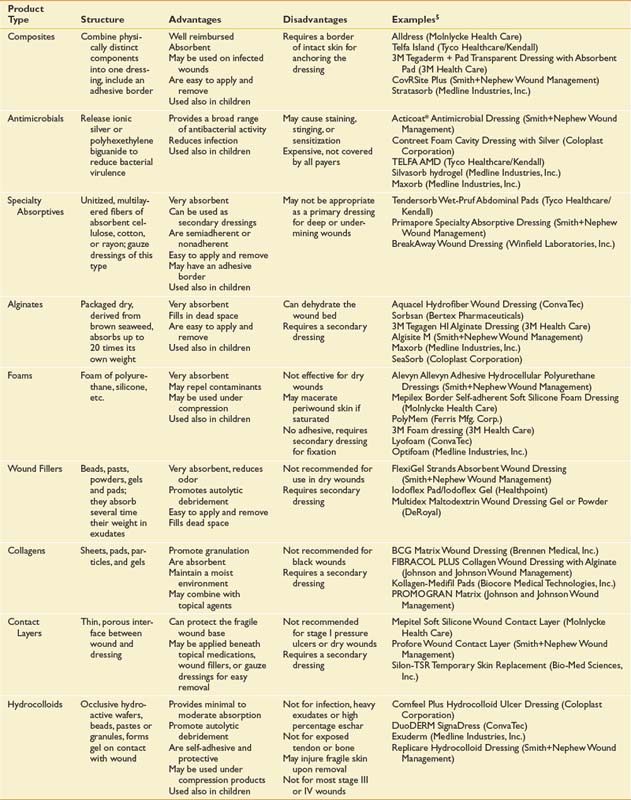
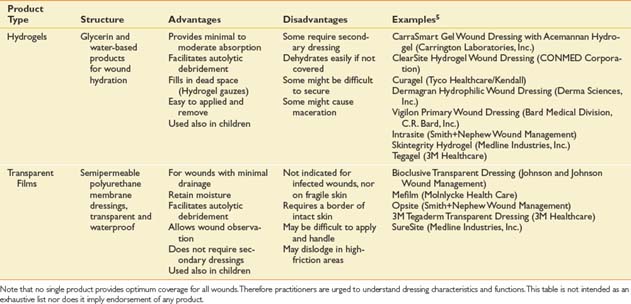
Dressings are typically applied in layers.102 The primary dressing is contiguous with the wound surface. A secondary dressing is applied external to the primary for absorption, protection, or fixation. For primary or secondary application, the dressing categories in Table 32-2 roughly correspond to the order in which they would be used in the process of wound bed preparation. The dressing categories in Table 32-2 are organized from most to least absorptive. Note the following caveats: (1) It is unusual to use more than a few categories for a given wound; (2) composites, specialty absorptives, and foams are more likely to be used for a longer period, or until healing; and (3) in small, clean stage II and III wounds, use of hydrocolloids, hydrogels, and films is the norm.
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) refers to systemic exposure of the body to 100% oxygen at a level higher than atmospheric pressure. The rationale of HBOT is to increase the amount of oxygen delivered to the wound area. Treatment significantly increases the oxygen concentration in the plasma but not the oxygen carried by hemoglobin molecules.113 Oxygen dissolved in the plasma can reach even poorly perfused wounds. Hyperoxic vasoconstriction in the surrounding normal tissue also diverts blood to poorly perfused wounds.194 Therapeutic effects at each stage of the healing process have been reported, such as increased collagen synthesis, improved bactericide, and reduction of leukocyte activation and adhesion on reperfusion of ischemic tissue.194 HBOT typically involves a series of daily “dives” in 100% oxygen at 2 atmospheres for a 4- to 6-week period. HBOT has been demonstrated to reduce the incidence of major lower limb amputations among persons with diabetes.103 Although the evidence supporting the efficacy of HBOT in treating chronic wounds is largely limited to diabetic wounds, HBOT also has been recommended for ischemic wounds and osteomyelitis.194
Gene Therapy and Exogenous Application of Growth Factors
Exogenous application of growth factors has been proposed as a means of promoting wound healing because these factors are often deficient in chronic wound environments. Despite the obvious rationale, clinical application of recombinant growth factors has been limited, partly because the growth factors tend to get digested by the proteolytic enzymes in the wound bed, and only a small fraction of these factors survive and diffuse into the wound tissue. Recombinant human platelet-derived growth factor BB (PDGF-BB) was approved by the Food and Drug Administration (FDA) for use in diabetic ulcers. For the recombinant PDGF to reach therapeutic levels in the wound site, however, a dose 50 times the minimum effective dose is required.121 Clinical trials testing the efficacy of recombinant human keratinocyte growth factor–2 and recombinant human granulocyte colony-stimulating factor are underway.127,171
Gene therapy refers to the insertion of a desired gene into recipient cells. The large surface area and superficial location of wounds renders the skin a good candidate for gene therapy. Gene therapy has been introduced to wound care practice for more efficient delivery of growth factors. Permanent insertion of DNA to the genome of recipient cells and transient transformation for short-term expression of a gene are two approaches to reach this goal.30 A variety of techniques, such as viral transfection, naked DNA application, liposomal delivery, and high-pressure injection, have been applied to facilitate the transfer of growth factor genes to the target wounds. High-pressure injection applied to cationic cholesterol-containing liposomal constructs, in particular, has shown great promise.29
Stem Cell Therapy
In the course of normal wound healing, multipotent stem cells in the bulge area of the hair follicle, epidermal stem cells in the basal layer of the epidermis, and the bone marrow–derived mesenchymal stem cells (MSCs) all participate in regenerating the cutaneous tissue.111,172 Bulge cells can reconstitute the epidermis, epidermal layers of the hair follicle, and the sebaceous glands. A subset of bone marrow–derived cells are found as dendritic cells and fibrocytes in the healed wound.41 In chronic, nonhealing wounds, dermal fibroblasts frequently display abnormal phenotypes and do not respond to PDGF-BB and TGF-β1.1,99 Stem cell therapy offers the possibility to remedy this abnormal wound healing milieu.
Autologous bone marrow–derived MSCs were cultured and applied topically to acute and nonhealing wounds in a study involving humans and murine models.69 A strong positive correlation was observed between the number of cells applied and the subsequent decrease in wound size. Randomized controlled trials to test the efficacy of these approaches and the exploration of the potential of multipotent bulge cells in stem cell therapy are anticipated.41
Phototherapy
Low-power laser in the visible or near-infrared (NIR) spectrum, ultraviolet light, and polarized light have been used in the clinical setting to foster healing of chronic wounds. Low-power laser therapy (LPLT) was reported to modulate inflammatory response, cellular respiration, and promote angiogenesis, fibroblast proliferation, collagen synthesis, and reepithelialization.4 Compared with visible light laser, NIR laser can penetrate deeper into tissues that are active during wound-healing processes.186 Polarized light therapy involves linearly polarized, polychromatic light containing infrared and visible spectrums. Polarized light can rearrange the polar heads of lipid bilayer in the cell membranes, thereby influencing cell surface enzymatic activities.58 Ultraviolet C light, which only reaches the upper layers of the epidermis, is noted for its bactericidal effect.63 In humans, the beneficial effects of LPLT in wound healing reported by small case series have not yet been reproduced in larger trials.161 A meta-study pooling two trials comparing LPLT with sham laser therapy on leg ulcers found no significant benefit of laser therapy (relative risk [RR] = 1.21; 95% confidence interval [CI], 0.73 to 2.03).51
Therapeutic Ultrasound
Ultrasound usage on wounds results in two types of therapeutic effect: thermal and nonthermal. Thermal effects are generated by high-intensity ultrasound and are manifested as increased tissue temperature. Thermal effect include increased blood flow and collagen extensibility.13 Nonthermal effects are largely due to acoustic streaming and cavitation. Streaming refers to the bulk fluid flow that can displace biomolecules and is due to the sound forces. Cavitation involves the formation and oscillation of microbubbles. Both streaming and cavitation appear to alter cell membrane activity, cell signaling, and cellular metabolism. Nonthermal effects of ultrasound include stimulation of protein synthesis, proliferation of fibroblasts and inflammatory cells, increased angiogenesis, collagen deposition and fibrinolysis, and release of cytokines and growth factors.13,178
High-frequency ultrasound (1 to 3 MHz) at intensities of 0.5 to 1 W/cm2 has been used in wound care, particularly on periwound tissues. A metaanalysis pooling four trials compared ultrasonic therapy with standard/sham therapies in the care of venous leg ulcers. The number of ulcers healed was greater (RR = 1.51; 95% CI, 1.09 to 2.09), and the percentage of remaining ulcer area was smaller (weighted mean differences = −5.34%; 95% CI, −8.38 to −2.30) in the ultrasound treatment group than in the control group.3 Therapeutic ultrasound for the treatment of pressure ulcers was compared with sham therapy in a metaanalysis pooling two studies involving 128 patients with pressure ulcers. No significant benefit of ultrasound on pressure ulcer healing rates was found (RR = 0.97; 95% CI, 0.65 to 1.45).51
The use of low-frequency (40 kHz), noncontact ultrasound was recently approved by the FDA for use in the treatment of wounds. Ultrasound is delivered to the wound bed via a saline mist without direct contact of the ultrasound transducer with the body. Mechanical energy at this frequency range is believed to exert debriding and cleansing action, as well as bactericidal activity through enhanced cavitation and streaming.178 Randomized, controlled trials have shown that this therapy accelerates healing and alleviates pain in chronic wounds of diverse causes.17,62,112
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree