CHAPTER 105 Postoperative Pseudomeningocele, Hematoma, and Seroma
PSEUDOMENINGOCELE
A pseudomeningocele is an extradural collection of cerebrospinal fluid (CSF) that has extravasated through a dural or arachnoid tear.1–3 Other terms used to describe this condition are ‘meningeal pseudocysts’ and ‘meningeal spurius’.4 Pseudomeningocele was first reported in the literature in 1946 following laminectomy for removal of a neoplasm.5
Epidemiology
Among patients undergoing primary decompressive lumbar spine surgery, incidental durotomy occurs at an incidence of 9–12%, making it the most frequently reported complication of lumbar spine surgery.6,7 After cauda equina syndrome, dural tears are the most frequent underlying diagnosis in malpractice litigation involving lumbar spine surgery.8
Pseudomeningoceles are a rare consequence of dural injury. In a review of 1700 laminectomies, Swanson and Fincher reported pseudomeningocele formation in four patients.9 In a group of 400 postlaminectomy patients with persistent back pain and/or radicular symptoms, Teplick et al. found pseudomeningoceles in 8 patients (2%).3
Factors that predispose to a dural tear in surgery are prior surgery or irradiation, congenital spinal malformations, unrecognized dural adhesions, dural calcification contiguous with overlying lamina, and the absence of epidural fat.8,10,11 Severe spinal stenosis and large herniated discs make root dissection and dural retraction more difficult and can predispose to dural tears. Dural injuries and pseudomeningoceles are understandably more frequent with posterior than anterior approaches.
Pathogenesis
Pseudomeningoceles generally develop following an intraoperative rent in the dura and arachnoid, but can occur following dural needle puncture procedures, especially after multiple punctures.9 The cerebrospinal fluid leaks dorsal to the lamina, into a space artificially created by dissection of the paravertebral musculature. The fluid cavity is lined by flattened connective tissue cells resting on loose, areolar connective tissue.2,4 An encysted pseudomeningocele refers to a CSF-filled herniation of an arachnoid lined cyst through a small rent in the dura. The narrow opening in the dura acts like a one-way valve leading to an increase in size of the CSF collection and formation of a pseudomembrane.2
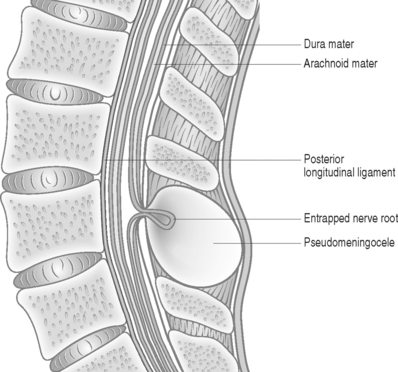
The size of the dural defect, pressure of the inflowing CSF, and the resistance of the surrounding soft tissues all influence the size of a pseudomeningocele. Postoperative weakness of paraspinal muscles may be a reason contributing to expansion of the pseudomeningocele.12 Spinal fluid pressure keeps the dural defect open and prevents healing. Herniation of nerve filaments into the original dural defect may be another factor responsible for keeping the dural opening patent (Fig. 105.1).4
Puncture of the dura can result in escape of large volumes of CSF, leading to intracranial hypotension and a demonstrable reduction in CSF volume. The adult subarachnoid pressure of 15 cmH2O can be reduced to 4 cmH2O or less. The rate of loss of CSF through a dural perforation following puncture with a 25-gauge or larger needle is generally greater than the rate of CSF production.13
Clinical features
Cerebrospinal fluid leaks after needle procedures in the lumbar spine classically present with headache. There is evidence that CSF leaks may be inevitable following needle puncture of the thecal sac,14 and it is unclear why only some of these patients are symptomatic. The size of the dural leak has no direct relationship with the occurrence of headache.14 The actual mechanism of headache is also unclear. Some authors suggest that the lowering of CSF pressure following a leak causes traction on the intracranial structures in the upright position. These structures are pain sensitive, leading to the characteristic headache.13 Others authors report that the drop in intracranial pressure causes a compensatory intracranial venodilation, which causes the headache. The headache is generally localized to the frontal and occipital regions, with occasional radiation to the neck and shoulders. Other symptoms may include nausea, vomiting, tinnitus, and vertigo.
While pseudomeningoceles may be asymptomatic, many present with local swelling, symptoms of CSF leak, or nerve root impingement. Local swelling15 may occasionally be the only suggestion of a pseudomeningocele.12 The swelling may vary in size, depending on whether the patient is standing or recumbent. There is no apparent correlation between the size of the pseudomeningocele, the size of the defect in the dura, and the degree of symptoms.4,16 Postural headache frequently occurs in patients with pseudomeningocele. Miller and Elder4 reported one patient whose postural headache could be increased by manual pressure directly over a large fluctuant meningocele.
Back pain, with or without radicular leg symptoms, is commonly associated with pseudomeningocele. In a review of 10 patients with postoperative pseudomeningocele, Miller and Elder found that back pain was a common feature in all patients, with nine having radicular pain radiating into the lower limbs.4 Manual pressure over the paraspinal musculature on the side of the cyst may exacerbate the back pain.16 Coexisting worker’s compensation issues can lead to doubts being expressed as to the authenticity of such complaints in the postoperative patient.16
Radicular findings in patients with pseudomeningocele result from herniation and kinking of the nerve roots in the dural defect,17 adhesion of the nerve roots to the edges of the sac,18 or direct pressure of the pseudomeningocele on an adjacent nerve root. Eismont et al.19 reported a case of persistent postoperative radicular pain along with focal deficits following lumbar disc excision. Myelography revealed a pseudomeningocele involving the left fourth and fifth lumbar nerve roots. Intraoperatively, it was found that the L4 root had buttonholed through the site of the dural tear, while the pseudomeningocele was found to be compressing the L5 root. Ossification of a pseudomeningocele can rarely cause neural compression.20 In many patients an obvious site of nerve root entrapment is not identifiable.4 Asymptomatic meningoceles can become symptomatic after a precipitating event leading to nerve entrapment.21 Radicular pain in a previously asymptomatic patient can be precipitated by maneuvers that increase intracranial and intraspinal pressure, such as coughing, sneezing, or jugular compression.16 Unusual presentations associated with pseudomeningoceles include bone erosion of the posterior vertebral elements22 and chronic meningitis.23
Investigations
Myelography shows a characteristic pattern of dye extruding through the stalk of the pseudomeningocele, and a fluid level may be visible when the opening in the dura is large. Percutaneous injection of contrast material directly into the cyst cavity has been carried out when a palpable or visible mass is present at a surgical site.16 CT-myelography can effectively demonstrate the neck and outline of the pseudomeningocele although nerve root entrapment is not well visualized.18
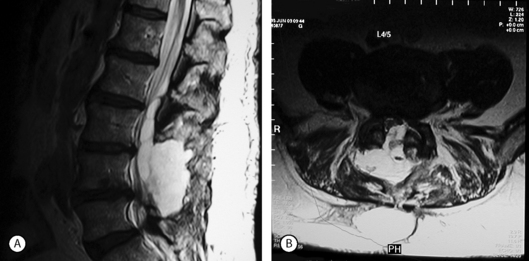
MRI scans are very sensitive for CSF collections and pseudomeningoceles (Fig. 105.2). Some authors recommend a routine postoperative MRI scan following any repair of a durotomy, even in the absence of symptoms of a pseudomeningocele, in order to ensure that no further leakage is present.24 Digital subtraction myelography may occasionally reveal a pseudomeningocele when plain myelography, CT scan, and MRI show a fluid collection posterior to the dural sac, but no obvious connection between the two.25 In radionuclide cisternography, radionuclide contrast material is injected into the subarachnoid space followed by MRI evaluation. Areas filled with CSF show up as high-signal regions.26
Management of dural tear and pseudomeningocele
In an attempt to reduce the incidence of postdural puncture CSF leak, numerous modifications have been made to spinal needles. Newer needle designs with narrow cutting tips and atraumatic bevels have resulted in a lower incidence of postspinal headache, neural irritation,13,27 and CSF leak.28
Spontaneous recovery from postdural puncture headache (PDPH) occurs in over 85% of patients within 6 weeks. Many forms of conservative management have been suggested and practiced, mostly without any scientific evidence to support their efficacy. Bed rest has been historically recommended, but does not lower the incidence of PDPH although it may delay its onset and decrease its intensity.29 Some authors suggest that bed rest may prevent further complications from traction and rupture of meningeal veins caused by intracranial hypotension.30 For severe and persistent headaches epidural blood patch (EBP) injections have been successfully used. Autologous blood is injected into the epidural space, where it forms a clot and seals the dural hole. Although over 90% of PDPH respond initially to EBP injections, a sustained and durable response has been identified in only 61–75%.31
Intraoperative durotomy is managed by primary dural repair, with a watertight suture of the dural defect. The key to prevention of postoperative pseudomeningocele is a meticulous primary repair of dural tears detected intraoperatively. Fine suture material such as 5-0 or 6-0 monofilament, braided or coated synthetic is suitable, although leakage of CSF can occur through the needle holes in the dura. A continuous running suture is quickest to perform, but may result in unraveling of the entire suture line if the tension is not perfect or the suture breaks. The use of magnification and coaxial illumination is essential for adequate repair. Local application of fibrin glue greatly reinforces a repair site.26,32
When a CSF leak is noticed postoperatively, surgical treatment is generally recommended for the immediate and definitive management of the lesion.19 Some authors recommend nonoperative management in a patient with a well-healed incision presenting with a soft subcutaneous bulge and no postural headache.33 Waisman and Schweppe treated seven patients by reinforcing the skin suture line, bed rest in the Trendelenburg position, and repeated puncture and drainage of the subcutaneous CSF collection. The CSF leakage stopped immediately after the incision was resutured, the subcutaneous fluctuation disappeared in 10–28 days, and an 8-year follow-up (clinical and ultrasound examination) revealed no pseudocyst formation.34 Eismont et al. reported one case where resuturing the skin stopped the CSF leakage through the incision. A symptomatic pseudomeningocele subsequently developed, and the authors recommends surgical repair of the dura for all dural leaks noted postoperatively.19
Closed subarachnoid drainage is frequently used in the management of CSF leaks33,35 and occasionally for established pseudomeningocele.36 The catheter is positioned in the subarachnoid space using fluoroscopic technique. Epidural catheter placement may be used for drainage of a pseudomeningocele.33 The proximal end of the catheter is connected to a sterile drainage system. The basis of this external CSF drainage is that a reduction of CSF pressure and preferential escape of the CSF through the catheter for 4–5 days generally allows the dural fistula to heal itself.
Lumbar peritoneal shunting may play a role in the management of refractory CSF leaks.33,37 The dural opening is surgically repaired when feasible. A catheter is then introduced into the subarachnoid space, and a second catheter left in the meningocele cavity; both catheters are subcutaneously tunneled into the peritoneal cavity, where they are positioned under the diaphragm using laparoscopic assistance. The advantages of this technique including immediate mobilization of the patient, avoidance of complications of external drains and repeated aspirations, and a high success rate. The procedure is not in widespread use due to technically simpler and less invasive options being available.
In previously irradiated tissue a vascularized myocutaneous flap can be rotated from an uninvolved area to the poorly vascularized defect and sutured to well-vascularized surrounding tissue. Newer techniques such as laser tissue welding are being investigated. Preliminary results show that primary repair combined with laser welding produces a higher leak pressure and tensile tissue strength than either technique used alone, with no evidence of underlying thermal tissue injury.38
Patients with established pseudomeningoceles who are not significantly disabled by their symptoms may be treated with observation alone. Follow-up MRI scans occasionally show complete resolution of small pseudomeningoceles.2 Sustained local pressure has been found to help control some symptoms of pseudomeningoceles. Leis et al.15 reported this form of treatment in a patient who was advised surgery but declined. The patient used a wide belt worn tightly around the waist and a folded towel under the belt so that it applied direct pressure over the swelling. This relieved the postural headache symptoms immediately. Repeat MRI scans done 8 weeks and 6 months after surgery showed progressive decrease in the size of the pseudomeningocele, till there was only a tiny focus of fluid left. The authors suggest a trial of focal compression for symptomatic relief of postural headache from pseudomeningocele. If symptomatic improvement is obtained, a more prolonged trial of mechanical compression may promote dural closure.15
Symptomatic pseudomeningoceles generally require surgical intervention.2,21 The operative procedure usually involves opening of the pseudomeningocele, followed by identification and closure of the dural defect with flaps from the cyst wall or a free graft. Augmentation of the repair with fibrin glue, muscle, fat, and fascial grafts is frequently necessary. Resection of overlying lamina is generally necessary for adequate visualization of the dural defect, and spinal fusion is indicated when excessive bone resection renders the spine unstable.4 Miller and Elder4 reported on the surgical treatment of 10 symptomatic pseudomeningoceles. The authors reported good results in seven patients, satisfactory results in two, and a poor result in one patient.
Apart from surgical repair, the only other surgical option for pseudomeningocele is CSF diversion, a technique that is not always successful, especially when spinal fusion implants prevent the normal reapproximation of paraspinal tissues around the collection. In addition, a ball-valve mechanism at the dural fistula site may prevent complete drainage of CSF from the pseudomeningocele. The cavity could persist and reaccumulate CSF after the drain is removed.39
Complications of untreated pseudomeningocele include recurrence of radicular symptoms, the possibility of infection with resultant meningitis,23 ossification of the cyst wall producing canal stenosis and claudication,40 and rarely erosion of the bony vertebral elements from an expansile cyst.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree