3 Post-stroke problems
Introduction
Approximately 50% of people who survive a stroke are left with long-term disabilities (Mackay and Mensah 2004) and stroke is the major cause of adult disability. Post-stroke problems may be the direct result of the stroke, indirectly related to the neurological event (e.g. due to reduced activity following stroke), or associated with the longer-term effects of disuse, inactivity and changes in lifestyle after stroke.
The purpose of this chapter is to raise awareness of the most common post-stroke problems and explore how they may affect a stroke survivor’s ability to engage in physical activity. It builds on the descriptions of post-stroke neurological deficits introduced in chapter 1, but goes into more detail, introduces the important longer-term post-stroke problems as well as co-morbidities, and considers their broad implications for exercise. The latter will be discussed in more detail in chapter 10, which focuses on the design of exercise programmes after stroke.
Problems with movement and functional activity after stroke
Motor impairments
As described in chapter 2, motor symptoms following stroke may include weakness, spasticity and contractures, which indirectly may lead to loss of range of movement.
Weakness
Weakness, also known as hemiparesis or hemiplegia, primarily affects the side of the body opposite to the side of the brain lesion. However, as will be detailed in chapter 4, it is important to note that the so-called ‘unaffected side’ is often also weaker than normal (Andrews and Bohannon 2000).
In the hemiparetic lower limb, weakness of the extensor muscles is common, which may cause difficulties with rising from a chair, walking and stair climbing (Saunders et al. 2008). Weakness of the muscles that extend the ankle and lift the forefoot (i.e. the ankle dorsiflexors) is common and can impair heel strike during gait, a problem known as a ‘drop-foot’. This is an important problem, as lack of foot clearance increases the risk of falls. As a compensatory measure, people with a drop-foot may be fitted with a splint or orthosis (e.g. an ankle-foot orthosis or AFO), while others may use an electrical stimulation device that controls ankle movement during walking (Burridge et al. 1998, Taylor et al. 1999).
In the upper limb, weakness may affect all muscle groups, including shoulder flexors and abductors, and elbow flexors and extensors, often making it difficult to reach and grasp objects. Weakness affecting muscles of the wrist and hand may impair hand function, including grip strength and dexterity (Boissy et al. 1999). Some people with stroke may be able to grasp an object, but uncontrolled activity of the finger flexors together with weakness of the extensors may make it difficult to release the object.
Spasticity
Spasticity1 is a complex topic. It is a motor disorder, characterised by abnormal muscle activation, which can be felt by both the stroke survivor and the therapist as increased resistance to movement when a muscle is passively stretched. In this book, we will use the definition from a European Union Consortium, which described spasticity as: ‘Disordered sensori-motor control, resulting from an upper motor neurone lesion, presenting as intermittent or sustained involuntary activation of muscles’ (Pandyan et al. 2005, p. 5).
Spasticity is common after stroke; around 38% of stroke survivors are estimated to develop spasticity over the first year (Watkins et al. 2002). In the acute stage after stroke, the brain is in a state of ‘shock’ and muscle activation may be temporarily absent, resulting in abnormally low tone or flaccidity. When the brain begins to recover, hyperactive reflexes and abnormal muscle activity emerge, often together with paresis. This combination makes it difficult for the stroke survivor to move and maintain an optimum posture. This can lead to further problems, as abnormally high tone together with weakness reduces range of movement. If this situation persists, connective tissue within and around the muscle will begin to shorten and form adhesions, thereby increasing the biomechanical stiffness of the muscle even further. Thus, disordered muscle activation is a direct result of the stroke, whereas changes in connective tissue are an indirect problem.
Entire patterns of muscle hyperactivity may be seen, known as ‘associated reactions’. For example, when a person with stroke tries to walk, the sheer effort may spark off hyperactivity in the upper limb, resulting in a flexor pattern involving the shoulder, elbow, wrist and hand (Fig. 3.1). When the level of effort reduces, the associated reaction also usually diminishes.
There are other factors that may trigger spasticity in people with a neurological condition, e.g. pain, an ingrown toenail, pressure sores or a urinary tract infection (Barnes 2001).
The term ‘spasticity’ is often used interchangeably with ‘high tone’ or ‘hypertonia’ (Edwards 2002). However, it is important to differentiate between these concepts, which are related but not the same. Loosely described, ‘tone’ reflects the tightness of a muscle or muscle group. Normally, muscle tone increases when we prepare for action, while it reduces when we relax. It is important to understand that muscle tone is a combination of neurogenic (i.e. muscle activation) and biomechanical factors. In fact, in fully relaxed muscles of healthy people, muscle activation may be entirely absent, and muscle tone is purely caused by biomechanical factors (Walsh 1992). These include the length and tension of connective tissue within and around the muscle. At a microscopic level, this includes cross-bridges between actin and myosin filaments within muscle fibres, which may be resolved through gentle, repetitive movement, which in turn reduces soft tissue stiffness (Walsh 1992). This phenomenon is known as ‘thixotropy’2 (Walsh 1992), a familiar analogy of which is stirring a pot of paint before using it to decorate a wall: the stirring action breaks the connections between the molecules and makes the paint more ‘runny’. Thixotropy is dependent on temperature and thus the soft tissue stiffness experienced by stroke survivors may be partially attributable to thixotropy caused by lack of movement and further compounded by decreased temperature caused by reduced circulation of the affected limb.
Traditionally, physiotherapists have been cautious about using exercise (particularly strength training) with stroke survivors, for fear that this might increase spasticity and affect motor recovery (Bobath 1990). However, a recent systematic review indicates that spasticity is not increased by exercise (Borges et al. 2009). Although the precise impact of exercise on spasticity is not fully understood, it is important that exercise professionals have a good understanding of this phenomenon in order to enable stroke survivors who have spasticity to engage with exercise safely and effectively.
Clonus is an involuntary, rhythmic contraction of a muscle that often sustains itself, having been provoked by a fast stretch (Sheean 2001).
Treatment
Treatment for spasticity is usually required when it interferes with function and hygiene, or causes pain, or when long-term complications are expected (Barnes 2001, Thompson 1998). Given that other factors (e.g. pain) can contribute to spasticity, these problems need to be carefully examined and treated where possible. Drug treatment may be either systemic (i.e. the drug affects the entire central nervous system, such as oral baclofen) or local (i.e. the drug affects a specific location only, such as phenol or botulinum toxin) (Ward and Ko Ko 2001). Systemic, oral anti-spastic medication may have unwanted side effects such as drowsiness (Ward and Ko Ko 2001), which could affect a person’s ability to exercise safely. Therefore, if spasticity is a local problem (e.g. mainly affecting wrist and finger flexors, or hip adductors), focal pharmacological agents tend to be preferred. Botulinum toxin type A is used increasingly, as there are usually few side effects, while its administration (i.e. by injection) is relatively straightforward (Davis and Barnes 2001). Other focal techniques include nerve blocks, which are undertaken by injecting alcohol into the nerve, or surgery by cutting through a specific nerve known to be the cause of the spasticity; however, these treatments are not routine and require further evaluation (Scottish Intercollegiate Guidelines Network 2010).
A number of different physiotherapy techniques are used to reduce hypertonia, although it is often not clear whether the intervention targets neurogenic, biomechanical, or both factors. Examples are electrical stimulation, stretching, splinting, positioning, or serial casting, whereby the position of a joint (e.g. the ankle) is increased in a step-wise manner to gradually stretch soft tissue around it (e.g. the plantar flexors of the ankle). However, there is no evidence that electrical stimulation (Pomeroy et al. 2006), stretching (Katalinic et al. 2010) or splinting (Lannin et al. 2007, Lannin and Herbert 2003) have any effect on spasticity, while there is insufficient evidence to support serial casting (Mortenson and Eng 2003).
Contractures
A contracture is a fixed deformity of a joint with a permanent loss of joint range of movement. This usually happens when joint movement has been reduced for some time. Contractures of the wrist flexors have been reported within 6–8 weeks of stroke (Pandyan et al. 2003). Contractures tend to be due to persistently increased muscle tone, permanent biomechanical changes in soft tissue around a joint, muscle weakness and inactivity. Contractures may cause pain and, in more extreme cases, secondary complications such as pressure sores at points of contact, e.g. the inner aspect of the knees in a stroke survivor with contractures of the adductors of the hip (Edwards 2002).
Problems with balance, sit to stand, walking, reaching and grasping, and swallowing
Balance
Stroke survivors may have difficulty maintaining their balance during sitting, standing and/or walking (Bonan et al. 2004) and as a result may be at risk of falling. Impaired balance may occur as a direct result of a stroke without limb weakness, or the paresis and/or sensory symptoms may cause people to be unsteady.
Sit to stand
Implications for exercise
Stroke survivors may need to be reminded to try and stand up symmetrically when rising from sitting, which in turn will help to activate weak leg muscles through visual, vestibular, proprioceptive and tactile feedback (Shumway-Cook and Woollacott 2007). The choice of chair height could also be considered as it will be easier to stand up symmetrically from a higher seat.
Walking (gait)
Around three-quarters of stroke survivors will walk independently, but on average at a much lower speed than normal (Ada et al. 2003). The symptoms of stroke can cause a variety of walking problems that may arise as a result of weakness, loss of sensation, abnormal muscle tone, lack of visuospatial awareness, impaired balance and coordination problems (Michael et al. 2005). Stroke gait is usually characterised by a short stance phase on the affected leg (i.e. the amount of time the foot is placed on the ground), coupled with a quick step with the stronger leg. People often fail to ‘step through’ with the stronger leg as it can then be difficult to relax the affected leg muscles sufficiently to take the next step (Woolley 2001). There may also be a degree of asymmetry of the trunk, usually side-flexed towards the stronger side.
Reaching and grasping
Earlier, we mentioned how weakness of the muscles around the shoulder, elbow, wrist and finger joints could affect the ability to reach, grasp and release objects. This may be compounded if there are soft tissue adhesions that limit range of movement, and/or spasticity affecting the ease and accuracy with which movement can be completed. In addition, there may be coordination difficulties that affect function of the arm and hand. Reaching in stroke survivors is slower, less smooth and more variable (van Vliet and Sheridan 2007), suggesting that more time is needed to take in the necessary visual and proprioceptive information and plan the activity step-by-step.
Implications for exercise
There are some typical compensatory movements that can be observed in stroke survivors, which include bending the trunk, often combined with elevating the shoulder girdle (Archambault et al. 1999). To maximise the training effect of arm exercises (Michaelsen et al. 2006), exercise professionals should emphasise an upright, symmetrical posture during reaching exercises and help stroke survivors avoid such compensatory movements. Exercise professionals should be aware that stroke survivors may not have sufficient grip force to hold objects in their hand and care must be taken, e.g. when working with a pole or weight.
Swallowing
As mentioned in chapter 1, more than half of stroke survivors may experience difficulties with swallowing (dysphagia) (Gordon et al. 1987), which is caused by reduced strength and/or coordination of muscles involved in chewing and swallowing, as well as potentially altered sensation in the pharynx. Dysphagia is a risk factor as people may choke when trying to swallow food or liquids. Some stroke survivors whose swallow has been affected can be given texture-modified diets (e.g. puréed food) and their fluids can be thickened to reduce the risk of aspiration (i.e. when secretions or foreign material enter the airways).
Common interventions to improve motor function after stroke
As described in chapter 2, treatment to improve motor function after stroke is based on a detailed, ongoing assessment of the individual and may involve input from the multidisciplinary team. Commonly used therapeutic strategies include specialist facilitation techniques to elicit muscle activity and improve strength and coordination (Baer and Durward 2004, Carr and Shepherd 2003, Jackson 2004, Raine 2009). In hydrotherapy, buoyancy of the water can be instrumental in facilitating movement in cases where muscle force is insufficient to act against gravity on land. Electrical stimulation or functional electrical stimulation (FES) may be used to induce or augment muscle contraction, e.g. to improve grasping (Popovic et al. 2005) or heel strike during walking (Burridge and McLellan 2000). Current practice involves intensive, functional, task-specific practice where possible (French et al. 2007, Langhorne et al. 2009, Steultjens et al. 2003, van Peppen et al. 2004). The rationale for this type of practice is based on the idea that each functional activity is subserved by dedicated neural networks. When practising often enough, these networks will become more efficient, finally leading to long-term neuroplastic changes (Carey et al. 2005, Nudo et al. 2000). Although evidence is still lacking in some areas of physical rehabilitation after stroke, examples of intensive, task-specific practice that are currently recommended (Scottish Intercollegiate Guidelines Network 2010) are constraint-induced movement therapy (Wolf et al. 2008), where the affected arm is forced to participate in functional activity as the other arm is prevented from being used for a considerable period each day (e.g. as the hand is placed in a mitt) and robotic devices that enable repetitive practice in purpose-designed activities (Mehrholz et al. 2008). Another example is gait training (either over ground or on a treadmill), which is recommended to improve walking (Scottish Intercollegiate Guidelines Network 2010).
Bladder and bowel problems after stroke
It is estimated that around 40–60% of patients with acute stroke have urinary incontinence and that this problem persists in 15% of stroke survivors at 1 year after stroke (Thomas et al. 2008). The first stage of managing incontinence is to identify and treat reversible causes, which may be unrelated to stroke. Sometimes a so-called ‘unstable bladder’ may contribute to the problems, and this may respond to drugs such as tolterodine. Regular toileting may also help and some stroke survivors who are unaware of the status of their bladder may have been advised to go to the toilet at specific times (a strategy known as ‘timed voiding’). Stroke survivors who still have urinary incontinence after careful assessment and specific treatment may require ‘pad and pants’ to contain the urine. Sometimes sheaths are used for men. As a last resort, a catheter may be inserted, but catheters carry a risk of infection and are generally avoided as a way to manage incontinence.
Problems with sensation and perception after stroke
As mentioned in chapter 1, 50–60% of stroke survivors have some form of sensory impairment, though the reported prevalence varies widely (Carey 1995). We will now provide more details on the most common sensory impairments after stroke and explore their potential impact on the ability to engage in exercise.
Visual problems
Following stroke, a large proportion of stroke survivors may have visual problems (Rowe et al. 2009). These may be a direct effect of the stroke itself, as will be explained below, or caused by premorbid factors. Many stroke survivors will have had significant visual problems prior to their stroke, including cataracts, age-related macular degeneration, glaucoma or diabetic retinopathy. Visual impairment may affect the ability to perform activities of daily living such as reading and driving, while they reduce quality of life and may increase the risk of falls, particularly in unfamiliar environments such as gym settings.
The type of visual impairment depends on the part of the visual pathway affected by the stroke (Fig. 3.2), and on pre-existing visual problems. Common visual impairments after stroke include double vision (diplopia), gaze palsy (difficulty controlling eye movements, due to paresis of extraocular muscles), visual inattention (also known as unilateral neglect, see below) and visual field defects, including homonymous hemianopia. Homonymous hemianopia is a condition whereby the same field (homonymous) of view is obscured (anopia or anopsia) in both eyes (Fig. 3.2). From chapter 1, we know that the left occipital lobe receives information from the right visual field and the other way round. Hence, if a stroke affects the left occipital lobe, the person is likely to have difficulty perceiving their right visual field in both eyes.
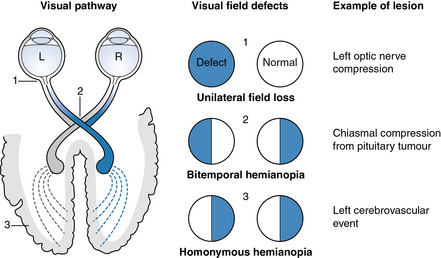
Fig 3.2 Three common visual field defects, including homonymous hemianopia due to a stroke.
Image from http://www.dwp.gov.uk/img/visual-stroke.jpg reused with permission and courtesy of Frank Munro.
The management of vision after stroke requires a multidisciplinary approach (Scottish Intercollegiate Guidelines Network 2010). For example, the physiotherapist and occupational therapist can provide exercises and advice on ways to manage visual impairments during activities of daily living, while ophthalmologists, orthoptists and opticians also play an important role in examining and prescribing treatments for visual impairment after stroke.
Implications for exercise
Exercise professionals need to know if a stroke survivor has visual problems, as this has implications for the stroke survivor’s own safety and that of others. Wherever possible, the exercise professional should position themselves in the stroke survivor’s preserved field of view, and ensure they can see objects and people around them. Chapter 9 will detail the risk assessments that the exercise professional needs to undertake prior to exercise, which involves removing clutter and ensuring adequate lighting to prevent falls.
Problems with tactile and proprioceptive sensation
Sensory function
Sensory modalities may be affected by stroke, causing sensory information to be absent, diminished, distorted or amplified. For the stroke survivor, sensory impairment can be disturbing. For example, some may experience a burning sensation in their hand when gripping an object. Sensory impairment may also be detrimental to motor recovery, for example upper limb function is hampered by severe sensory impairment despite the presence of active movement (Carey 1995, De Souza 1983). This is because sensory information is required to coordinate fine, skilled movement such as writing or doing buttons.
Stereognosis
Occasionally, stroke survivors with right hemisphere damage can present with a sensory problem known as astereognosis. People with asterognosis may be unable to recognise objects through touch alone (i.e. with vision occluded) (Turvey et al. 1998) and may have difficulty with using equipment and dressing. Treatment for this problem includes allowing the individual to examine objects thoroughly through touch and using this sensory information to provide clues to the objects used (e.g. examining a cup with a handle would allow the individual to identify a key characteristic about the object, indicating how this would be used).
Disorders of thermal sensation
Stroke survivors with any type of stroke can experience disordered perception of warm/cool and/or hot/cold sensation in comparison with healthy people (Choi-Kwon et al. 2006). This may not be immediately apparent as people may not be aware of it themselves. Disorders of thermal sensation may also be associated with the development of central post-stroke pain (Bowsher 2001). Some stroke survivors with disturbed thermal sensation can experience pain when touching normally non-painful stimuli, such as cold metal; this is known as allodynia.
Disorders of taste
About 30% of stroke survivors have reduced taste after a stroke (Heckmann et al. 2005). This may affect appetite which partially explains poor nutritional status following stroke (Poels et al. 2006). Whether improvements in taste occur in the longer term is uncertain.
Unilateral neglect
Also known as unilateral neglect, contralateral neglect, visuospatial neglect or hemi-inattention, unilateral neglect is a phenomenon whereby a person fails to attend to, perceive stimuli in, or move towards their affected side (Manly and Robertson 2003). Following a stroke, a person may be unaware of auditory, visual or tactile stimuli on the side opposite to the lesion (i.e. the affected side). They may fail to notice objects or people on their affected side and walk into them. They may also fail to notice that their affected side (e.g. their arm) is in an unsafe position and at risk of being harmed. There are a number of possible causes for this complex problem, including disorders with attention (see below), reduced sensation or perception of the affected side, or a combination of these factors. Neglect is particularly commonly found in people with a large stroke affecting the right hemisphere.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






