Chapter 24 Peripheral Joint and Soft Tissue Injection Techniques
Overview and History
The first published description of intraarticular corticosteroid injection was by Hollander et al.79 in 1951, who demonstrated clinical improvement with the injection of hydrocortisone in a case series of patients with inflammatory joint conditions. Hollander78 subsequently reported on 250,000 injections in 8000 patients and was the first to describe complications associated with intraarticular corticosteroid injections. These included postinjection flare, aseptic necrosis in weight-bearing joints, and infection (infections occurred at a frequency of 1 in 15,000 injections). Practitioners have since expanded the use of corticosteroids to include injections near tendon insertions for enthesitis and other periarticular structures such as bursae.183 These injections have other known complications including localized fat atrophy and tendon rupture.25 Despite these known complications, physicians continue to use corticosteroids as evidenced by a survey showing that 89% of physiatrists171 and 93% of rheumatologists use intraarticular and soft tissue corticosteroid injections for the treatment of pain.75 Fortunately, other medications such as local anesthetics and hyaluronan derivatives are available that can also be used to help with musculoskeletal pain conditions when corticosteroids are not indicated or are not beneficial.
Purpose: Diagnostic or Therapeutic
Intraarticular space or soft tissue injections can be used either for diagnostic or therapeutic purposes. Obtaining fluid from a joint, bursa, or cystic structure can help determine the cause of a fluid collection. Although a complete discussion is beyond the scope of this chapter, certain laboratory studies and bedside analyses can help categorize the nature of the fluid (Table 24-1). Cell count classifies the type of fluid as being noninflammatory, inflammatory, infectious, or septic. Culture and sensitivity help in the identification of a specific organism and its susceptibility to antibiotics. Birefringence classifies the type of crystals present. Although laboratory analysis is the most specific and sensitive way to analyze aspirated fluid, the string test allows for a nonspecific bedside screen for inflammation. Because of its highly viscous nature, healthy synovial fluid should be able to stretch at least 2.5 cm before breaking when dripped from a syringe. Inflammation decreases the overall viscosity and makes the fluid drip instead of stretch. If the aspirated fluid is noninflammatory, it should be clear enough to read 0.25-inch font through a test tube.134 The fluid should be sent for formal analysis when an infection or a crystal arthropathy is suspected, or when the cause of the fluid collection is uncertain.92
Although aspiration can help classify the etiology of the fluid, injecting an anesthetic into a structure might aid in identifying or confirming whether the structure is a pain generator.94 For example, a diagnostic injection into the hip joint using fluoroscopic guidance can help distinguish whether pain is emanating from a pathologic disorder of the hip joint or is referred from the lumbar spine. Because single anesthetic diagnostic injections have a known false-positive rate, a second confirmatory injection might be necessary.45
Not only can injections be used for diagnostic purposes, they can also be effective in the treatment of painful conditions. Although isolated aspiration of accumulated fluid can be helpful in some cases, any benefits obtained from solely removing fluid are often short-lived.201 Consequently various injected medications and preparations have been used alone or in combination to provide a longer therapeutic benefit for various musculoskeletal conditions. Performing these injections should help lessen the need for oral medication, improve function, and reduce disability. Optimally injections should not be performed in isolation, but in combination with physical therapy and exercise.26 Injected medications such as anesthetics, corticosteroids, and various preparations of viscosupplementation have different adverse effects, durations of action, and costs. These factors should be thoroughly understood before use of injections in the clinical setting.
Medications
Corticosteroids
Mechanisms, Types, and Adverse Effects
Corticosteroids are commonly used for intraarticular or soft tissue musculoskeletal conditions and have multiple mechanisms of action. Although corticosteroids can cause various systemic effects, the antiinflammatory and immunosuppression properties are due to the glucocorticoid effects. Although glucocorticoids act directly on nuclear steroid receptors to control the rate of synthesis of messenger ribonucleic acid and proteins in T and B cells, they also produce changes in white blood cell traffic and alterations in levels of cytokines and enzymes, and inhibit the function of phospholipase A2. The overall effect of these reactions is a reduction in proinflammatory derivatives such as bradykinin, histamine, prostaglandins, and leukotrienes. Bradykinin and histamine are capable of directly stimulating primary afferent nociceptive fibers, whereas prostaglandins and leukotrienes sensitize nociceptors.37 One objective measure of these antiinflammatory effects is the reduction of erythrocyte sedimentation rates and C-reactive protein levels.188 In addition to the effect on inflammation, corticosteroids have also been reported to have a direct stabilizing effect on neural membranes and inhibit C-fiber transmission, thereby reducing ectopic discharges from neural fibers, including those from within the spinal cord.128,150 This could help explain how corticosteroids might have antinociceptive effects even in noninflammatory conditions.86 These antiinflammatory and antinociceptive effects are often delayed, explaining why the therapeutic onset of corticosteroids can take 24 to 48 hours to produce any benefit.206
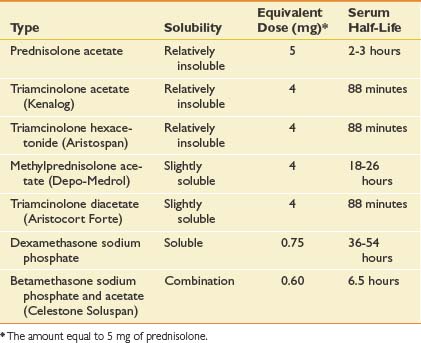
Multiple corticosteroids are available, each varying in potency, solubility, and duration of action. In one survey of rheumatologists, methylprednisolone acetate was the most frequently used corticosteroid at 35%, followed closely by triamcinolone hexacetonide (31%) and triamcinolone acetonide (22%). Each corticosteroid preparation has known properties pertaining to potency, relative doses, half-life, and solubility (Table 24-2).
Corticosteroid solubility is a key feature that varies among the preparations available. It is postulated that corticosteroids with lower solubility (such as depot preparations) might remain at the injection site longer and maintain higher effective synovial levels.34 In fact, corticosteroid depot preparations have such low solubility that their crystals have been found at the site of an injection up to 1 month postinjection.63 Because of their lower solubility and greater duration of action, there can be a greater concern that injecting depot corticosteroids around soft tissue structures might lead to local adverse effects.34 This is in contrast to more water soluble corticosteroids like dexamethasone, which can diffuse away more easily and potentially create more systemic effects.34
Intraarticular injections have generally demonstrated an excellent safety record and are described by the American College of Rheumatology as “safe and effective when administered by an experienced physician.”10 Corticosteroid injections do, however, have known adverse effects. A postinjection flare can occur in 2% to 6% of patients63 and is thought to be the result of a chemical synovitis from the injected particulate corticosteroid crystals.81,116 Although this postinjection flare typically occurs within 24 hours, it is usually self-limited and can be effectively managed with ice packs, analgesic therapy, or in rare cases, aspiration of joint fluid.164 Corticosteroids can also cause fat atrophy and skin changes, but at a rate less than 1%.97 There are case reports of less common adverse effects, including tendon rupture with direct intratendon injection∗ and avascular necrosis.3,56,67,72,127 Recent evidence has also shown that patients who receive intraarticular corticosteroids in the 6 months preceding total joint arthroplasty have an increased risk of infection postoperatively.43,104,138,200
Corticosteroids also have known adverse systemic effects. Facial flushing occurs in up to 15% of patients, and has an onset within hours and can linger for several days.140 Intraarticular corticosteroid injections have also been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis.99,135,157 Although usually mild and transient, prolonged HPA suppression can last up to 11 weeks and in one case has caused Cushing’s syndrome.135 Other systemic effects include increased hepatic glucose synthesis and antagonism of insulin for up to 2 weeks after the injection, potentially worsening any preexisting hyperglycemia.132 Any potential effect on bone metabolism appears to be transient and mild, without known clinical relevance.49 There have been no case reports of corticosteroid-induced myopathy after intraarticular injections.
There is a concern that intraarticular injections can cause joint degradation because of the direct catabolic effect of the corticosteroid, or because of the increased use of the less painful but still diseased joint. In rabbits, corticosteroids have been shown to damage chondrocytes,137 but this effect has not been confirmed in primates.60 In a prospective trial comparing intraarticular injections of either saline or triamcinolone every 2 months for up to 2 years in patients with knee osteoarthritis, there were no differences in joint space narrowing seen in either group at the 2-year follow-up.154 In another study in patients with rheumatoid arthritis, there was no difference in joint arthroplasty rates between those who received four or more intraarticular injections annually and those receiving less frequent injections.159 In children with juvenile rheumatoid arthritis, intraarticular injections of corticosteroids did not appear to affect cartilage integrity.82,182 Because of the known and unknown adverse effects, it is generally accepted that physicians should limit the total number of corticosteroid injections to three or four annually per patient.10,143
Efficacy
Most reviews andTM large prospective studies have demonstrated a positive efficacy of corticosteroid injections in inflammatory conditions at the shoulder,12,22 hip,58,151,213 and knee joints.11,14,62,213 There are additional uncontrolled trials and reports for small joints23,123,205 and soft tissues70,91,142,162,172 that mostly consist of heterogeneous studies with small patient populations, differing disease pathology, and varying methodological quality.14 When taken as a whole, the literature demonstrates that corticosteroids may provide short-term pain relief for various musculoskeletal conditions.8,108,113,158 Currently the American College of Rheumatology guidelines recommend intraarticular corticosteroid injections for selected patients with signs of inflammation.10 Too few studies are available to make a recommendation for a specific corticosteroid preparation (i.e., triamcinolone vs. betamethasone).213
Carette et al.26 studied the effect of fluoroscopically guided corticosteroid shoulder joint injections on symptoms from adhesive capsulitis. Patients were randomly assigned to one of four groups: corticosteroid injection and physical therapy, corticosteroid injection alone, saline injection and physical therapy, and saline injection alone. At 6 weeks the group that received the corticosteroid injection and physical therapy had significant improvements in pain and range of motion, but by 1 year all four groups had similar results. The data suggested that a corticosteroid injection helped pain in the short-term but did not affect long-term outcomes.
Studies using US-guided subacromial bursa corticosteroid injections have demonstrated variable efficacy.29,31,48,129,161 No studies to date have analyzed the efficacy of US-guided corticosteroid injections into the biceps tendon sheath, glenohumeral joint, or acromioclavicular (AC) joint.
Corticosteroid injections have been studied extensively for carpal tunnel syndrome.8,108,113 A prospective study of surgical decompression versus local corticosteroid injection in patients with carpal tunnel syndrome resulted in symptomatic relief in both groups followed up for 1 year.108 A Cochrane Review looked at 12 studies of carpal tunnel syndrome and determined that “local corticosteroid injection provides significantly greater clinical improvement than oral corticosteroid for up to three months,” including improvements in pain and paresthesias.113 There also could be long-term improvement in hand function and electrophysiologic studies.8
Corticosteroid injections can be considered a definitive treatment for certain conditions, such as de Quervain’s tenosynovitis.158 Kullenberg et al.96 conducted a prospective, randomized, blinded trial comparing the use of either 80 mg triamcinolone acetonide or mepivacaine 1% in patients with osteoarthritis who were awaiting hip replacement surgery. Injection with corticosteroids under fluoroscopic guidance resulted in the greatest improvement in pain and function at 3 weeks and 12 weeks. The group that received mepivacaine did not show significant improvement in either parameter at 3 weeks and withdrew from the study before 12 weeks because of lack of effect. Robinson et al.160 compared 56 patients with hip pain injected under fluoroscopic guidance with 40 mg of methylprednisolone, and 36 patients with hip pain injected with 80 mg of methylprednisolone, with follow-up at weeks 6 and 12. When the doses were compared, the group that received the 80-mg dose demonstrated a significant improvement compared with the 40-mg group at 6 and 12 weeks. Imaging findings did not relate to severity of symptoms or response to injections. Plant et al.146 studied the effect of 80 mg of methylprednisolone and lignocaine on pain at the hip from osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. Fluoroscopic guidance was used to confirm needle placement in the hip joint. At 12 weeks those with some evidence of a hypertrophic (visible osteophytes) pattern on plain radiographs had a significant response to the injection.96 In a controlled pilot study, Qvistgaard et al.151 showed that patients treated with repeated corticosteroid injections into the hip using US guidance had significant improvement during the study period, which indicated a moderate clinical effect.
Several studies have shown that intraarticular corticosteroid knee injections provide 1 to 4 weeks of relief of pain for osteoarthritis of the knee.11,37 Sustained relief has not been demonstrated in the literature. There is a paucity of studies examining the efficacy of various conditions with US-guided knee injections. Bliddal20 compared the efficacy of US-guided injections of methylprednisolone with that of etanercept in multiple joints of patients with rheumatoid arthritis. Although a subgroup analysis was not completed specifically for the knee, no significant differences were found between the two groups for the relief of pain. Another uncontrolled trial showed good short-term outcomes when US guidance was used to aspirate and inject corticosteroids into perimeniscal cysts.109
Viscosupplementation
Mechanisms, Types, and Adverse Effects
In osteoarthritis the hyaluronic acid found in synovial fluid decreases in both molecular weight and concentration. Because of this reduction, injectable hyaluronan derivatives (collectively known as viscosupplementation) were developed for therapeutic purposes. Although their exact mechanism of action is unclear, some theories do exist including possible antiinflammatory or antinociceptive effects, or stimulation of in vivo hyaluronic acid synthesis.207 Restoration of the viscoelastic properties of the synovial fluid appears the most logical explanation, but other mechanisms must exist because studies show that the injected hyaluronic acid only remains in the joint space for hours to days.32 In fact, an optimal effect might only develop after repeated weekly injections, but can persist for several months.194
Adverse reactions associated with these medications are uncommon, and no long-term systemic effects have been reported.155 The most common adverse reaction reported is a pseudoseptic reaction that occurs 24 to 72 hours after the injection, but at a rate of less than 3%.185 This reaction should be differentiated from the much less frequent complication of an infected joint.
Efficacy
The literature on the efficacy of intraarticular viscosupplementation is primarily focused on the knee, with limited studies of other joints. Although the literature covering viscosupplementation for knee osteoarthritis has been conflicting and has a known publication bias,15,105 metaanalyses suggest an improvement in pain compared with placebo.105 In patients who have failed conservative treatment, there is evidence that viscosupplementation is beneficial for the treatment of knee pain caused by osteoarthritis.15,105 In one large placebo-controlled, randomized trial of 660 patients with moderate to severe shoulder pain caused by osteoarthritis, rotator cuff tear, or adhesive capsulitis, a significant improvement in pain relief was found in all groups up to 13 weeks after viscosupplementation.18 Thirty patients with symptomatic osteoarthritis of the shoulder who failed conservative treatment and were treated with intraarticular viscosupplementation demonstrated improvement in function and pain levels.170
Although viscosupplementation has been studied extensively and with high-quality studies for knee osteoarthritis,105 few high-quality studies have evaluated its use in the treatment of hip osteoarthritis. Van den Bekerom et al.194 performed a systematic review of 16 studies, with only two high-quality studies of viscosupplementation injections for hip osteoarthritis using fluoroscopic or US guidance. In the level 1 study that was done, the efficacy of low-molecular-weight viscosupplementation was compared with that of high-molecular-weight viscosupplementation but not with placebo.190 Patients had significant improvements with both types of viscosupplementation for up to 6 months. Because the placebo effect can be significant,105 these results should be viewed cautiously. In uncontrolled trials using different US approaches, repeated viscosupplementation injections demonstrate significant improvement in visual analogue scale (VAS) and functional outcomes.24,124,125,149,191 Despite the lack of high-quality research, viscosupplementation has been shown to improve symptoms in uncontrolled studies and can be considered on an individual basis.
Anesthetics
Mechanisms, Types, and Adverse Effects
Local anesthetics are frequently used in intraarticular and soft tissue injections to not only decrease the pain from the injection but also for diagnostic purposes. There are two classes of anesthetics, the esters and the amides. They differ in solubility, pH, and vasodilatory effects, resulting in unique onset of action, potency, and duration (Table 24-3). These properties can be altered by the addition of other agents such as epinephrine and sodium bicarbonate. Epinephrine and sodium bicarbonate can increase the pH, thereby prolonging the duration of action of local anesthetics. Epinephrine acting via vasoconstriction prolongs the duration of action and decreases systemic absorption.98
| Agent | Duration of Action | Maximum Dose (per Procedure) |
|---|---|---|
| Esters | ||
| Procaine (Novocain) | Short (15-60 minutes) | 7 mg/kg; not to exceed 350-600 mg |
| Chloroprocaine (Nesacaine) | Short (15-60 minutes) | |
| Amides | ||
| Lidocaine (Xylocaine) | ||
| Mepivacaine (Polocaine, Carbocaine) | 7 mg/kg; not to exceed 400 mg | |
| Bupivacaine (Marcaine) | ||
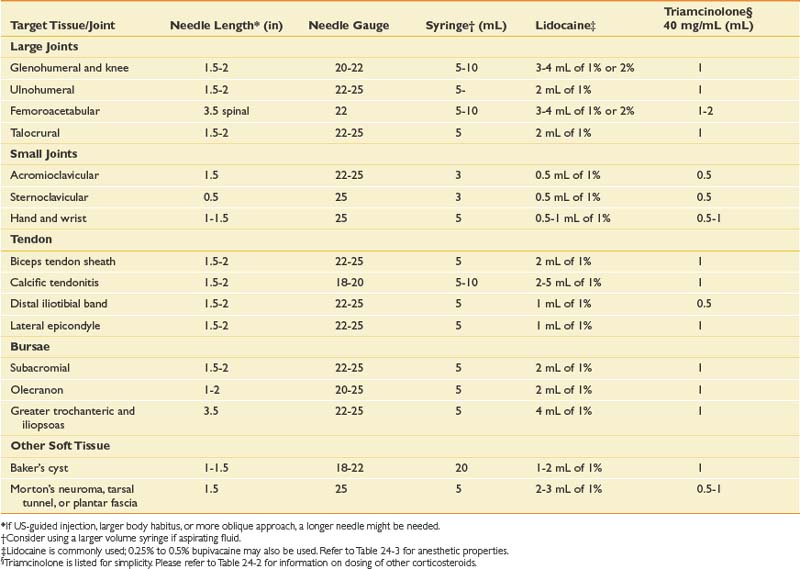
Anesthetic dosage is determined by the target tissue and the desired level of local anesthesia (Table 24-4). Increasing the dose shortens the onset of action and increases the potency and duration, but also increases the possibility of an adverse reaction.98
Anesthetics can have adverse effects on the central nervous system, cardiovascular system, and immune system. Cardiovascular effects include direct myocardial depression and bradycardia, which can lead to cardiovascular collapse. Patients who have renal or hepatic compromise, are older or pregnant, or who have preexisting cardiac disease can be at an increased risk of toxicity. The biggest cause of toxicity is direct intravascular injection, making it necessary to routinely aspirate before injection. Lower concentrations of local anesthetics are typically used for joint and infiltrative soft tissue injections (see Table 24-4). Given the small doses for joint injections and these lower concentrations, systemic toxicity should be exceedingly rare.
The esters are derivatives of para-aminobenzoic acid and have been associated with significantly higher rates of allergic reactions than the amide anesthetics. For this reason, most physicians prefer using amide anesthetics.98 Recent animal and in vitro studies have demonstrated a dose- and time-dependent toxicity on cartilage that has not been demonstrated in vivo.44,90,167 There is also a concern that local anesthetics might be directly neurotoxic.95
General Considerations
Before any injection, several factors must be appropriately addressed, including the equipment needed and obtaining informed consent. Specific needle type, length, and gauge will vary depending on the target tissue, type, and volume of fluid to be injected or aspirated (see Table 24-4). Informed consent should include all potential risks and benefits of the procedure, including possible alternative treatments. The risk profile will vary depending on the medication given, and should be adjusted accordingly. Aside from the previously mentioned adverse effects of the medications, there are also risks associated with insertion of a needle through the skin, including infection, bleeding, hematoma formation, and neurovascular injury.
Joint infections are rare, with an incidence from 1 in 3000 to 1 in 50,000.28 Symptoms typically occur 3 to 4 days postinjection, whereas postinjection flares usually are reported within the first 24 hours.163 Infection can be minimized through the use of sterile technique.
Bleeding can be minimized through proper technique, the use of epinephrine in the local anesthetic, and assessing for any bleeding diathesis. Patients receiving warfarin only have a mild increased bleeding risk if the international normalized ratio is at an appropriate therapeutic level. For this reason some practitioners state that it is not typically necessary to discontinue warfarin before a peripheral joint injection.189
Non–Image-Guided Injections
Most practitioners do not use any image guidance for the majority of peripheral joint injections. The overall accuracy of a non–image-guided “blind injection” varies between joints, approach, and experience. Overall the literature demonstrates a 10% to 40% “miss rate” of blind injections when compared with US-guided or fluoroscopic-guided injections. Although the use of either fluoroscopic or US image guidance has been shown to enhance the accuracy of injections, it has not been conclusively proven that enhanced accuracy is related to an enhanced efficacy.68
Ultrasound-Guided Injections
With improved technology and subsequently better resolution, musculoskeletal US has been increasingly used to help guide peripheral joint and soft tissue injections.4,7,87,177,202
Unlike other imaging modalities, US has the unique advantage of being able to visualize soft tissues, bony landmarks, and the needle with real-time scanning, thereby allowing dynamic visualization.4,7,87,177
Unlike fluoroscopy, which uses radiation and primarily visualizes bone and joints, diagnostic US is unique because it uses acoustic waves, which are safe in all patient populations. US offers superb soft tissue resolution, surpassing that of magnetic resonance imaging for certain conditions.130 With this superb resolution, nearby neurovascular structures can be localized and avoided during any injection. Doppler imaging not only helps identify normal vasculature, but also aids in identifying the neovascularization that has been associated with pathologic conditions such as tendinopathy.46 Studies have suggested that US-guided destruction of these neovessels results in clinical and morphologic improvements.76,103,212
The biggest disadvantage of using musculoskeletal US is that it is largely operator dependent, requiring proper technique to adequately visualize and inject underlying structures.84,118,195 Most injections using US are performed with the “free hand technique,” where one hand holds the probe while the other hand positions the needle.7,87,180 Because of this, performing US-guided injections requires manual dexterity and has a documented learning curve.141 It is highly recommended that before attempting US-guided injections, individuals gain experience with diagnostic US imaging and have an understanding of its capabilities and limitations. Injection experience can be obtained by practicing on phantom models or participating in instructional courses; however, these are not equivalent to hands-on supervised training.
A strong knowledge of musculoskeletal anatomy and the ability to discern pertinent structures with diagnostic US are essential. An important step that helps in identifying these structures is selecting the most appropriate transducer or probe.84,177 For example, high-frequency (5 to 12 MHz) linear array transducers are helpful for imaging more superficial structures, whereas low-frequency (3 to 5 MHz) curvilinear transducers might be needed for injections of deeper structures such as the hip joint.102,175 The “hockey” stick probe with its smaller footprint, however, might better enable visualization of superficial structures near the wrist and ankle (Figure 24-1).177
It is recommended that a preliminary scan be carried out before performing any US-guided injection. This preliminary scan serves several purposes. The first purpose is to identify any neurovascular structures that need to be avoided during the procedure. The preliminary scan also allows adjustment of the depth, focus, gain, and time-gain compensation so that optimal visualization of the intended target can be obtained.84,177,196,197 The decision can then be made regarding the best approach, with marking of the planned entry site.84
For each US-guided injection, various approaches can be used, each having advantages and disadvantages.7,118,180 The chosen approach is often dependent on the practitioner’s comfort level, training, and experience. Because there are numerous approaches to injecting musculoskeletal structures, the techniques described below are merely suggestions, and crucial concepts will be highlighted.
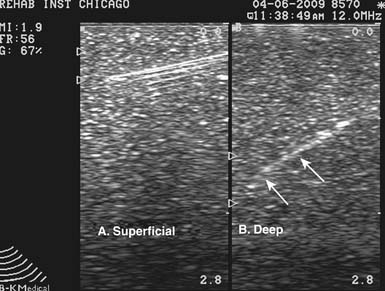
US can image an injection needle in longitudinal or transverse planes.7,177 When the longitudinal view is used, the transducer lies parallel to the needle. This is often referred to as the needle being “in plane” with the transducer.177 With the short axis or transverse view of the needle, the transducer is oriented perpendicular to the needle, and the needle is considered to be “out of plane” (Figure 24-2).177 Because a longitudinal view of the needle should yield consistent visualization of the needle shaft and tip, this is the preferred approach and is especially useful when first learning US-guided procedures.177 The out-of-plane technique makes identifying the needle tip difficult because the shaft will appear the same anywhere it is viewed along its length. To identify the needle tip, the acoustic outline of the bevel must be visualized, which can be challenging.4,87,177,181 It cannot be stressed enough that when performing an US-guided injection, it is crucial that the needle tip be constantly visualized.87 Without constant visualization of the needle tip, injury to nearby structures and inaccurate placement of medication can occur.
Proper visualization of the needle tip can sometimes be improved by slightly spinning or by jiggling the needle gently to see perturbations of the nearby tissue, with or without the use of Doppler imaging. Hydrodissection can also lead to better identification of the needle tip. With this technique a small amount of sterile saline or local anesthetic can be injected during the US-guided procedure.7,177 The relatively hypoechoic fluid provides a darker background compared with the relatively hyperechoic needle, making the needle more conspicuous.
Certain factors affect the brightness or echogenicity of the needle, including needle depth and obliquity, with needle gauge being less important. When the US beam does not strike the needle at a 90-degree angle, some of the beam will be reflected away from the transducer, and the needle will appear less bright or hypoechoic.7,84,118,177,196 Consequently, it is ideal to keep the transducer and US beam perpendicular to the needle to make it as bright or hyperechoic as possible.7,84,177 Deeper tissues often require the needle to be directed at an oblique angle, resulting in the US beam striking the needle at an angle other than 90 degrees. Regardless of its gauge, the needle will appear less echogenic with a more oblique approach, and visualizing the needle while injecting deeper tissues can be more difficult (Figure 24-3). These injections might be better suited for the more experienced ultrasonographer.7
Injecting very superficial targets such as tendon sheaths, cystic structures, and some joints can also be technically demanding. When an intended target lies close to the skin surface, there is often not enough subcutaneous tissue to place an injection needle under the transducer. This can be circumvented by using a standoff approach. With this technique, sterile US gel is heaped up on the skin under the probe overlying the superficial target. Then the injection needle is placed directly into the gel, providing initial visualization under the probe and eventually guided down into the intended target (Figure 24-4).4 Essentially the standoff gel serves as a vehicle that helps to place the needle under the probe near the superficial structure without having to traverse the needle through tissue. Angling the transducer as needed serves to improve the echogenicity of the needle even before its entering into the skin.
FIGURE 24-4 Standoff demonstration: acromioclavicular joint injection with ultrasound guidance using hockey stick probe.
Because needle echogenicity is highly dependent on technique, commercially prepared echogenic needles might not be worth the added expense. It is much more important that the needle be visualized perpendicular to the US beam. With this in mind, a 27-gauge needle can be as easily recognized on US as some larger gauge needles.87,177
Although there are no absolute recommendations as to whether to perform these injections with sterile technique, every attempt at minimizing the risk of infection should be undertaken.4 Commercially prepared packets that contain both sterile US gel and sterile transducer covers are available. Although this might limit the risk of infection, patients can perceive such an elaborate setup as somewhat overwhelming. Other patients will be reassured knowing that this injection will be placed accurately, because the needle and pertinent tissue will be visualized during the entire procedure. Although some US-guided injections can be performed with the patient in a seated position, having the patient lie down can help ease any anxiety and also mitigate a potential vasovagal reaction. Proper technique includes gripping the transducer while maintaining good contact with the patient’s skin. This reduces hand fatigue and, more importantly, provides tactile feedback to the clinician.177 Although no absolute standard exists, there are guidelines that recommend appropriate documentation of the study.131 This can include archiving preinjection and postinjection images, as well as images that verify needle position at the time of the actual injection.
Technical Guide
Upper Limb Techniques
Glenohumeral Joint
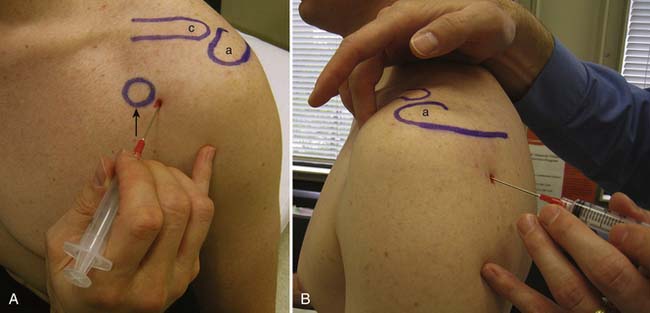
There are multiple ways to perform blind glenohumeral joint injections.51,69,148,168,169 Among these techniques, the accuracy of an anterior approach ranges from 27% to 99%, and a posterior approach ranges from 50% to 91%. When a blind posterior approach is used, the patient is seated with the ipsilateral hand and forearm resting on the lap. A 1½- or 2-inch needle then is placed into the skin 1 to 1½ inches below the posterolateral aspect of the acromion (Figure 24-5). The needle is then advanced anteriorly and slightly medially toward the coracoid process until it is felt to rest within the joint. For the anterior approach, the needle is inserted just lateral to the coracoid process and advanced posteriorly and slightly medially (see Figure 24-5). The typical volumes used for glenohumeral joint injections are found in Table 24-4. In cases of adhesive capsulitis, the overall volume of the glenohumeral joint is reduced, but injecting as much volume as tolerated will help in breaking up the adhesions limiting the range of motion and contributing to this condition.193
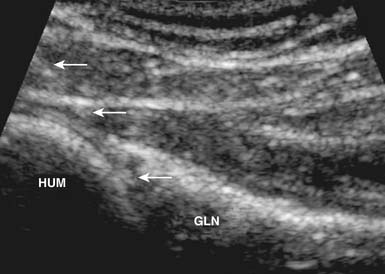
With the use of US guidance, the glenohumeral joint can be injected with the patient prone and the affected arm hanging off the table, or alternatively with the patient side-lying with the affected side up. A posterior short-axis approach provides the best access into the joint.4,102 The needle enters the skin in plane with the transducer from a lateral approach and enters the joint posterior to the humeral head (Figure 24-6). Because of the relative depth of this joint, a spinal needle often is needed to perform this injection.4,55
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree