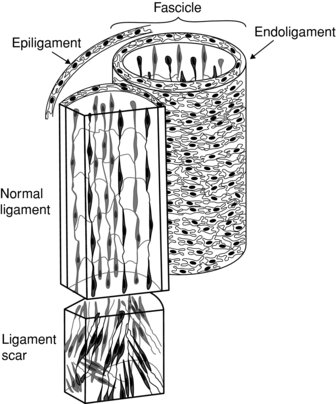
Each ligament is built by multiple fascicle units. Each fascicle carries the collagen fibrils and fibroblasts cells in longitudinal lines and encapsulates them in a loose connective tissue called endoligament. Fascicles are separate enough to allow shearing movements occurring at different joint’s position (Frank 1996). The entire ligament is covered with a vascular epiligament, a loose connective tissue that does not undergo much tension during the ligament’s tightness (Ian et al. 2002).
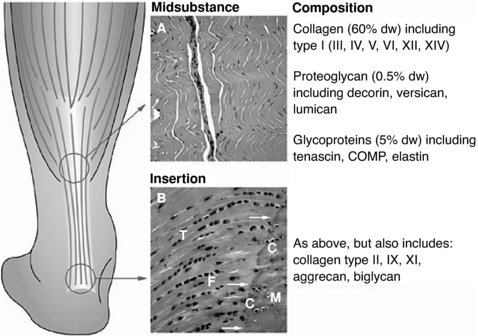
The enthesis is the insertion of a ligament to the nearby bone and has a special arrangement (Figure 6.2). This is where the collagen fibres fan-out and are attached diagonally to bones via the periosteum. This arrangement helps in dissipate the ligament stress more evenly to the bone during different joint’s position (Benjamin et al. 2006).
Figure 6.2 The enthesis. Reprinted, with permission, from Nature Clinical Practice in Rheumatology (2008) 4, 82–89 doi: 10.1038/ncprheum0700 © Nature Publishing (2008)
One character of the fascicle is the “crimps” – a waviness of the fibrils. The crimps give the ligaments its pseudo-elasticity and disappear once the ligament is stretched (Scott 2003).
Many ligaments are attached to the joint capsule, tendons and other connective tissue and cannot be separated anatomically and functionally, hence the common injuries where more than one tissue is damaged.
Blood supply
The blood supply to ligaments deserves a special examination as in most cases it is the limiting factor in healing injured ligaments. Compared with other tissue, ligaments are hypovascular and their blood supply is better closer to the bones’ attachments; the middle section is poorly supplied (Bray et al. 1996).
The blood supply to ligaments may arrive from three places. The epiligament carries blood along the ligament, where blood vessels branch out to the endoligament and inside the fascicles. A build up of tension in the ligament will reduce the amount of blood circulating, but will recover at rest (Bray et al. 1996). The periosteal blood supply supplies mostly the enthesis region of the ligament. The surrounding connective tissues such as fat, joint capsule or muscles may carry some blood that collaterally supplies ligaments (Arnoczky 1985; Bray et al. 1996).
Following injury, the vascularity increases for about 40 weeks while the ligament’s fibres become disorganised. The increased vascularity allows healing. The final post-injury state is a reduced vascularity (compare to the pre-injury level) with poor vessel organisation in the scar tissue. Ligaments probably do not have the ability to keep the original vascular organisation, causing reduced vascularity in the chronic healing stage, which may be the causes of higher level of reinjures.
Nerve supply
Ligaments carry two types of sensory impulses to the central nervous system: mechanoreceptor and pain. Mechanoreceptors signal mechanical events occurring in the tissue and have an important role in the coordinated motion pattern. Ruffini receptors are the most common mechanoreceptors in ligaments and joint capsule, whilst the others are Pacinian, Golgi tendon organ-like and free nerve endings. All of these receptors allowing the central nervous system to assess the amount of stress joints undergo and execute patterns of muscle contraction to help protect joints over stretching (Riemann and Lephart 2002b).
Following an injury both the peripheral and central nervous system undergo modifications.
One study found that the post-injury instability of the anterior crutiate ligament is due, in part, to remodelling of the central nervous system. The remodelling is probably due to the habitual reduction of usage of that area (Valeriani et al. 1996). As ligaments become strained and torn so the nerves supply loses a certain amount of receptors. This by itself can damage the peripheral nervous system and prevent it from accurately suppling the central nervous system with a real-time sensation of what happened to the joint.
In any case, following an injury it is probably both the central and peripheral nervous systems that are damaged and so supply corrupted sensory input to the central nervous system. This, in turn corrupts the muscular contraction pattern (Riemann and Lephart 2002b). One author’s hypothesis is that some cases of back pain, for example, may originate from chronic ligamentous tension, which sends a corrupt sensory message to spinal muscles to contract continuously, causing those muscle to be in chronic tension and fatigue (Panjabi 2007).
Physiology
Ligaments have similar mechanical properties to other connective tissues: viscoelasticity and stress-strain (Norking and Levangie 2005).
The first mechanical property of a ligament is its non-linear stress-strain relationship (see Figure 6.3).
Figure 6.3 Stress-Strain graph. Reprinted from Mow, V.C. and Hayes, W.C. ‘Basic Orthopaedic Biomechanics’ (ISBN: 9780881677966) © 1991, Lippencott, Williams and Wilkins
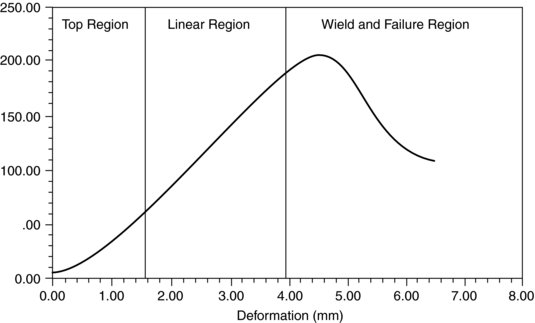
The stress-strain graph (Figure 6.3) demonstrates three regions:
1. Toe region – this is where the deformity (ligament length) is high while the force applied is low. Anatomically, this is where the fibrils’ crimps slack begins to tighten. The toe region is where most ligaments are at rest.
2. Linear region – this is where a stress build up creates a linear build up of strain or stretch to the ligament. This region demonstrates ligaments during a normal joint’s movement.
3. Failure region – this is where even a mild increased stress to the ligament creates a large deformation as the ligament is overstretched or torn.
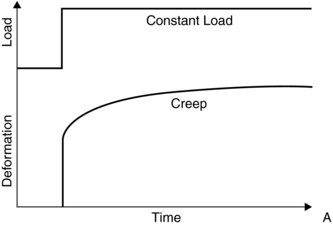
The stress-strain graph never perfectly occurs as the ligament also exhibits viscoelasticity property. Viscoelasticity is a tendency of the tissue to stretch and return slowly to its normal form whilst dampening the shearing force. There are three viscoelasticity tissue behaviours: creep, stress- relaxation and hysteresis.
Creep is the tendency of slowly increasing the ligament’s deformation under a load and the return to normal shape once the load is taken away (Figure 6.4). For example, under a load the ligament will slowly elongate, and then return to its original length once the load is taken away. The rate of creep increases at high temperature.
Figure 6.4 Ligament creep. Reprinted from Mow, V.C. and Hayes, W.C. ‘Basic Orthopaedic Biomechanics’ (ISBN: 9780881677966) © 1991, Lippencott, Williams and Wilkins
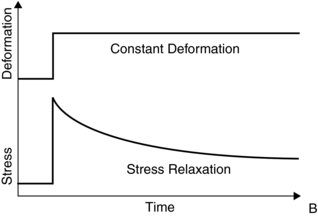
The second viscoelasticity property is stress relaxation (Figure 6.5). This means that under a constant deformation the stress will be reduced. For example, if a ligament will elongate during load, the stress will be reduced compared to a situation where the ligament will stay at a constant length.
Figure 6.5 Stress relaxation relationship in ligaments. Reprinted from Mow, V.C. and Hayes, W.C. ‘Basic Orthopaedic Biomechanics’ (ISBN: 9780881677966) © 1991, Lippencott, Williams and Wilkins
The third viscoelastistic property is hysteresis (Figure 6.6). Under load, ligaments undergo deformation that does not immediately return to the original length after unloading the joint. The difference between the length before loading and after unloading represents the amount of energy lost in the process. If, for example, the joint is loaded and unloaded many times, the stress-strain curve would move to the right, showing an increase strain to the same stress.
Figure 6.6 Hysteresis. Reprinted from Mow, V.C. and Hayes, W.C. ‘Basic Orthopaedic Biomechanics’ (ISBN: 9780881677966) © 1991, Lippencott, Williams and Wilkins
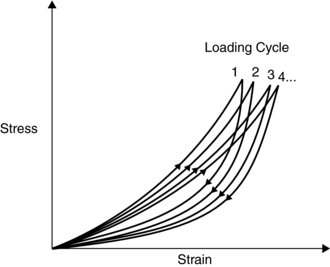
Figure 6.6 illustrates how loading a ligament many times in a short period can cause a strain or a tear if there is not enough recovery time between the cycles.
Normal changes to ligament through life
Ligaments exhibit adaptation to external and internal changes.
The effect of exercise and long-term immobility to ligaments are known and directly relate to the property of collagen fibrils to increase its thickness in response to exercise and reduce thickness in response to immobility (Kannus et al. 1992).
There are no differences between pre-puberty male and female ligamentous structure. However, following puberty females show an increased joint laxity (Quatman et al 2008). Before puberty the rate of anterior crutiate ligament (ACL) injury is equal male to female, but the post-puberty injury rate is at least 3-fold higher in adolescent females (Agel et al. 2005).
The knee crutiate ligaments express the sex hormone receptors for oestrogen, testosterone and relaxin (Faryniarz et al. 2006). There is an increased risk of ACL strain during the pre-ovulation stages of menstrual cycle, where hormone levels change from a low to high level (Slauterbeck et al. 2002). Due to different individual hormonal profiles during menstrual cycle there is a significant difference to the amount of ligamentous laxity and risk of injury (Shultz et al. 2006).
In the elderly there is an increase in joint capsule and ligaments laxity, which may be one of the reasons for the increase in incidents of osteoarthritis (Rudolph et al. 2007).
Pathology
Pathological changes in ligaments may occur due to structural and functional failure. Any strain to a ligament may cause a long-term joint instability. There are agreed degrees of ligaments strain: 1st degree is mild, 2nd degree moderate and 3rd degree is a complete tear (Chen et al. 2008).
Following a strain, ligaments do not heal by producing an identical tissue; instead, a scar tissue is formed. The scar tissue presents with an uneven matrix, smaller in diameter collagen fibers, weaker collagen crosslinking and a limited creep (Figure 6.7).
Figure 6.7 The healing ligament and creating of a scar tissue. Reprinted from Mow, V.C. and Hayes, W.C. ‘Basic Orthopaedic Biomechanics’ (ISBN: 9780881677966) © 1991, Lippencott, Williams and Wilkins
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







