Fig. 5.1
Acute osteomyelitis: Neutrophils infiltrate through the fibrous periosteum (arrow on periosteum) and into the cortical bone
Bone is a dynamic organ. Normal, healthy bone is composed primarily of lamellar bone (Fig. 5.2). Osteoblasts lay down unmineralized osteoid in layers, or lamellae. Osteoblasts surrounded by bone are called osteocytes, and they reside within spaces called lacunae. The osteocytes communicate with each other via cytoplasmic processes called canaliculi. Osteoclasts are responsible for breaking down the bone by a process of resorption. Together the osteocytes, osteoblasts, and osteoclasts remodel the bone in response to mechanical forces, calcium and phosphate levels, as well as other hormones and cytokines.
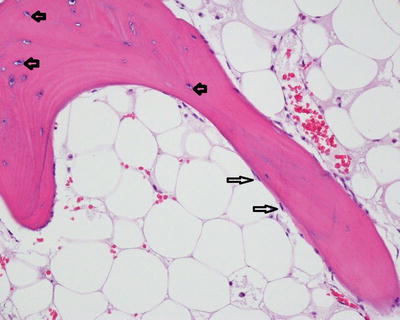

Fig. 5.2
Normal bone: Mature lamellar cancellous bone composed of layers of mineralized osteoid matrix. Inactive osteoblasts on the surface of the bone trabeculae (long arrows). Osteocytes within the bone matrix reside within lacunae (short arrows). The marrow space is filled with mature fat
When an infection breeches the periosteum and reaches cortical bone, osteoclastic resorption occurs causing scalloping of the cortical surface (Fig. 5.3). Pathogens and neutrophils gain access to the medullary canal via Haversian systems in the cortical bone (Fig. 5.4) and the host response of osteoclastic bone resorption continues. Because a bone is virtually a closed system, the edema from the inflammatory response increases the intramedullary pressure, impairing the blood supply further. Microscopic bone necrosis is evident after approximately 48 h (Fig. 5.5) [19]. Left untreated or partially treated, the process becomes chronic and gross necrosis may become evident. The term sequestrum refers to an area of segmental osteonecrosis which has become separate from the adjacent viable bone and may be identifiable in imaging studies [16]. As bone necrosis continues, the inflammatory activity stimulates the production of osteoblastic new woven bone formation between the periosteum and cortex (Fig. 5.6), termed the involucrum, which encases the sequestrum and strengthens the bone in the damaged area, much like a fracture callus.
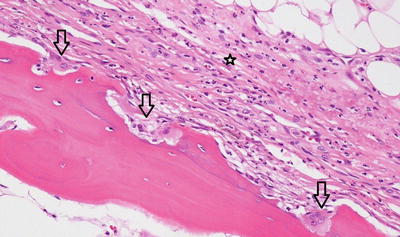
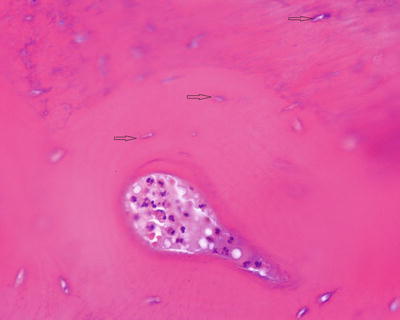
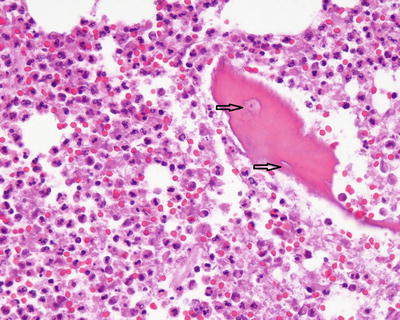
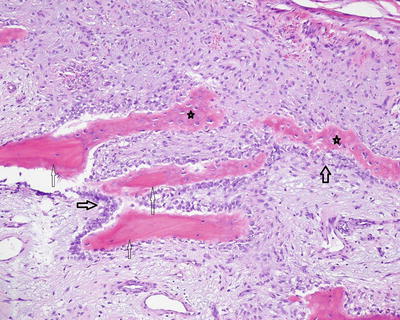

Fig. 5.3
Subperiosteal scalloping of cortex by activated osteoclasts (arrow osteoclasts). Neutrophils infiltrate the fibrous periosteum (star)

Fig. 5.4
Acute osteomyelitis: Neutrophils within a Haversian system of cortical lamellar bone. The bone is necrotic so most of the lacunae are void of osteocyte nuclei (arrows)

Fig. 5.5
Acute osteomyelitis: Necrotic trabecular bone surrounded by neutrophils. Note the absence of viable osteocyte nuclei within lacunae due to bone necrosis (arrows)

Fig. 5.6
Subperiosteal new woven bone with osteoblastic rimming may be produced in osteomyelitis and other conditions (dark arrows on activated osteoblasts). The woven bone (stars) is haphazardly arranged and lacks the lamellae, or lines, present in mature lamellar bone (thin arrows on lamellar bone)
The key histologic features of acute osteomyelitis are neutrophils in close association with necrotic bone, and osteoclastic activity with bone scalloping. Bone necrosis is recognized by the absence of osteocytes within lacunae (Fig. 5.7). Other histologic features may also be present such as marrow edema, marrow necrosis, and intravascular thrombi (Fig. 5.8). As the inflammatory process continues, osteoblasts produce woven bone to rebutress trabeculae of necrotic lamellar bone in a reparative process referred to as creeping substitution. Septic arthritis is characterized by acute inflammation involving the joint capsule, synovium, or damaging the articular cartilage (Figs. 5.9 and 5.10).
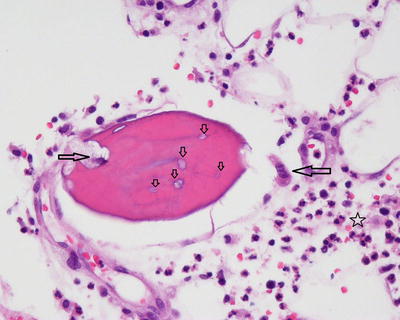
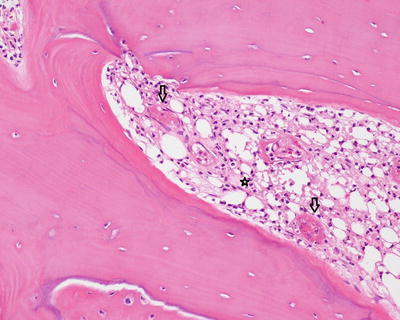
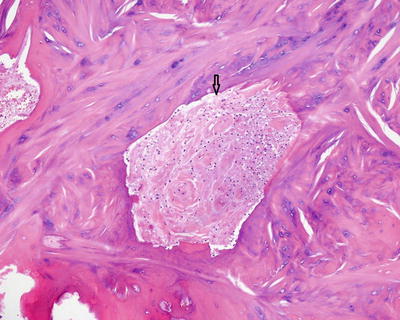

Fig. 5.7
Acute osteomyelitis: Necrotic bone with osteoclastic resorption (long arrows). The lacunae are devoid of osteocyte nuclei (short arrows). The marrow space contains abundant neutrophils (star)

Fig. 5.8
Acute osteomyelitis: Intravascular thrombi within the marrow space composed of pink, granular fibrin (arrows). Abundant neutrophils fill the marrow space (star)

Fig. 5.9




Acute inflammation involving fibrocartilage of a tendon insertion site (arrow neutrophils)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








