Reference
Classification
Overview
“Waldvogel”
• Mechanism
– Hematogenous
– Contiguous
– Vascular compromise
• Duration
– Acute
– Chronic
“Cierny-Mader”
• Anatomic
– Medullary
– Superficial
– Localized
– Diffuse
• Physiologic
– Normal host
– Compromised
– Prohibitive
[121]
“Buckholtz”
1. Wound induced
2. Mechanogenic infection
3. Physeal osteomyelitis
4. Ischemic limb disease
5. Combinations of 1–4
6. Osteitis with septic arthritis
7. Chronic osteitis/osteomyelitis
Type | Characteristics | |
|---|---|---|
Mechanism of bone infection | Hematogenous | Seeding of bacteria from a distance source that spreads through the bloodstream |
Contiguous | Infection from an adjacent site such as open fracture | |
Associated with vascular compromise | Infections in patients with peripheral vascular disease or diabetes | |
Duration of infection | Acute | Initial diagnosis of osteomyelitis. Edema, abscess, vascular congestion, small vessel thrombosis |
Chronic | Prolonged or recurrence of acute case | |
Ischemia, necrosis, sequestra |
Stage | Name | Characteristics | Clinical example(s) | |
|---|---|---|---|---|
Anatomic type | I | Medullary | Infection restricted to the bone marrow | 1. Infected intramedullary rod |
2. Hematogenous osteomyelitis | ||||
II | Superficial | Infection restricted to outer cortex | Diabetic foot ulcer with infection extending to bone | |
III | Localized | Well demarcated, full-thickness lesion without instability | Progression from Stage I or II | |
IV | Diffuse | Infection spread to entire bone circumference with instability | Progression from Stage I, II, or III | |
Physiologic class | A | Normal host | No comorbidities | |
B | Bs | Systemic compromise | Diabetes, malnutrition, renal failure, hepatic failure, malignancy, extremes of age, immune disease | |
Bl | Local compromise | Smoking, chronic lymphedema, major or small vessel compromise, venous stasis, arthritis, large scars, neuropathy | ||
Bls | Systemic and local compromise | Combination of above factors | ||
C | Prohibitive/poor clinical conditions | Treatment has a higher risk than osteomyelitis itself | Patient who is not a surgical candidate or who cannot tolerate long-term antibiotics |
Pathophysiology and Microbiology
The pathophysiology of osteomyelitis is complicated but a basic understanding can help in the diagnosis and treatment of this disease. Throughout the natural course of osteomyelitis osseous changes occur, biofilm forms, and neutrophils cause major defects. All forms of osteomyelitis begin by bacteria adhering to the bone matrix via receptors to fibronectin, fibrinogen, laminin, collagen, and proteins [4, 34–37]. The attached organisms cause an inflammatory response of the bone. As inflammation persists and intramedullary pressure rises, the vascular channels become obliterated causing patches of ischemia and bone necrosis. These areas of necrotic bone can detach from the bone and are called sequestra [4, 25, 26, 34–38]. As necrotic bone is forming, osteoclastic activity is stimulated by inflammatory factors such as interleukin-1 and tumor necrosis factor. This causes loss of bone and creates a destructive appearance of the bone. At the same time, a periosteal reaction begins and causes new bone formation. This surrounds and encases the sequestrum and is termed involucrum. During the process of bone formation and destruction cloaca form, which are openings in the involucrum that connect to the sequestrum. It is through the cloaca which exudate often extrudes [3, 38].
Bacteria are able to fend off host defenses as well as antibiotics through the formation of biofilm, and thus infections can persist even after medical or surgical treatments. Biofilms are colonies of pathogens that bind to the surfaces of wounds or implants. They are generally composed of 25–30 % pathogen and 70–75 % self-secreted amorphous matrix [34–39]. A wound bed is an ideal environment for biofilm to form since it is moist and nutritionally supportive. Biofilm also tends to form on devitalized tissue and bone, such as involucrum [38]. It has been reported that as rapidly as 10 h, many of the bacteria flora present on the skin can form a biofilm [40]. They generally are polymicrobial in nature with anaerobes, Serratia, Staphylococcus, and Pseudomonas being the most robust [38–41]. In addition to multiple species present, there are various mechanisms by which a biofilm inhibits wound healing and can make the host more susceptible to osteomyelitis. The matrix created by the biofilm itself creates a physical barrier that inhibits host inflammatory cells from ridding the body of the pathogens. Biofilms are highly resistant to antibiotics as they do not easily penetrate through this matrix. Also, there is a metabolically senescent nature of biofilm, which leads to further resistance since many antibiotics target rapidly dividing bacteria [35, 39, 41]. Thus, it has become increasingly important to treat and extinguish the biofilm in a wound, on the surface of hardware (screws, plates, suture, joint implants), or on exposed bone in order to fully treat or prevent osteomyelitis.
Most foot and ankle osteomyelitis is polymicrobial in nature, except hematogenous osteomyelitis, which is almost always monomicrobial [38]. As with soft tissue infections, the causative agent in bone infections is primarily bacterial but can also result from fungal, parasitic, viral, or mycobacterial infections (Table 1.4) [32, 42]. Staphylococcus aureus is the most prevalent causative organism in osteomyelitis [4, 43]. It accounts for the majority of hematogenous osteomyelitis in children and adults and is present in many other foot and ankle cases. S. aureus has a number of unique traits that make it particularly virulent. First, it contains factors that allow it to attach to extracellular matrix proteins contributing to early colonization of the host. S. aureus also has features such as toxins and capsular polysaccharides that make it less susceptible to host defenses. Osteolysis has been seen to occur rapidly from the increased osteoclastic activity due to the release of tumor necrosis factor-α, prostoglandins, and interleukin-1. It is the combination of these factors that makes S. aureus a common culprit in chronic infections leading to osteomyelitis [25, 26, 32, 35, 38]. Of great importance in foot and ankle osteomyelitis is the increasing prevalence of methacillin-resistant S. aureus (MRSA). This pathogen is frequently encountered in hospitalized patients along with other multidrug-resistant organisms. In 2013, it was reported that the incidence of community acquired MRSA was 1.6–29.7 cases per 100,000 and 2.8–43 % of those were bone and joint infections [44]. It has also been reported to account for 15.3 % of osteomyelitis cases in diabetic foot infections [26, 45, 46]. This rise in incidence throughout the general population, not just diabetic patients, has prompted clinicians to use broad-spectrum antibiotics prior to culture results. The treatment of MRSA osteomyelitis can be more prolonged and complicated with increasing lengths of hospital stays and complications [44, 45].
Common clinical setting | Etiology |
|---|---|
Hematogenous, all ages | Staphylococcus aureus |
Hematogenous, infants/children | Haemophilus influenzae |
Diabetes mellitus, vascular insufficiency, contaminated open fracture | Polymicrobial: Staphylococcus aureus, B–Hemolytic Streptococci, Enterococci, aerobic gram-negative bacilli |
Orthopedic implant devices, hardware, foreign bodies | Staphylococcus aureus, coagulase-negative staphylococci (Staphylococcus epidermidis) |
Human or animal bites | Pasteurella multocida, Eikenella corrodens |
Puncture wounds on the foot | Pseudomonas aeruginosa |
Soil contamination | Clostridium sp., Bacillus sp., Stenotrophomonas maltophilia, Nocardia sp., atypical mycobacteria, Aspergillus sp., Rhizopus sp., Mucor sp. |
Sickle-cell disease | Salmonella sp. |
Intravenous drug users | Pseudomonas aeruginosa, Staphylococcus aureus, Candida sp. |
Pseudomonas aeruginosa is another common organism seen in osteomyelitis of the foot. It is frequently seen as the infecting organism in plantar puncture wounds since it is present on the soles of shoes and its predilection for warm, moist environments. It has been reported that osteomyelitis complicates 1.8–6.4 % of puncture wounds sustained to the feet [2, 26, 47–49]. In 2.5–14.6 % of diabetic foot osteomyelitis, P. aeruginosa has been isolated and is associated with a higher rate of recurrence and amputation than S. aureus [26, 46]. Thus, P. aeruginosa may be a more problematic and underappreciated organism in osteomyelitis.
Populations at Risk
Osteomyelitis behaves differently in various patient populations as well as different anatomical locations. There are cohorts of patients that are at a higher risk of developing a bone infection and situations where the clinician needs a higher index of suspicion for the disease. Recognizing patients and clinical situations with a high predilection for developing osteomyelitis will help the clinician with early diagnosis and an appropriate treatment protocol.
As mentioned previously, hematogenous osteomyelitis most frequently occurs in children. Those with an even higher risk factor are children with sickle-cell disease [50]. Due to obstruction and damage to the spleen, they are at an extreme susceptibility to infection. Risk factors in adults include intravenous drug use as well as common causes of bacteremia. These include urinary tract infections, indwelling catheters, central intravenous lines, and hemodialysis [2].
Recent trauma or surgery can put a patient at a higher risk of developing osteomyelitis. Any foot and ankle surgery can lead to a deep infection involving the bone. An incisional dehiscence, if not treated appropriately and in a timely fashion, can cause a debilitating infection in the bone. Likewise, implanted devices including plates, joint implants, and external fixators bring a higher risk factor simply by introducing a foreign material into the body. These implanted devices due to its contact on the bone surface can provide an optimal environment for biofilm formation, which in turn can cause infection of the underlying bone [51]. Patients who sustain an open fracture are more susceptible to osteomyelitis until the bone is covered with a soft tissue envelope. The longer the bone is exposed, the more likely the chance of developing a complication [52]. It is recommended that definitive soft tissue reconstruction take place within 7 days of injury and ideally by day 3, to minimize the risk of reconstructive failure or deep infections [52–54]. Injuries to the nail plate can also increase the risk of bone infection, particularly in pediatric patients because of the lack of soft tissue between the nail and the underlying bone [2, 55, 56]. Puncture wounds to the foot as well as animal or human bites can predispose the bone to infection [48, 49].
Complicating factors such as peripheral neuropathy, peripheral vascular disease, and underlying immunocompromise can lead to foot ulcerations. Wound chronicity can eventually lead to deep ulcers that penetrate to the level of the bone. It is important for high-risk patients, such as diabetics, to minimize ulcerations by appropriate foot care and prevention [22, 23, 57]. Peripheral vascular disease (PVD), which is encountered in diabetic patients as well as tobacco abusers, is another risk factor. With decreased blood circulation to the foot or ankle, patients are at a higher risk of developing a wound [58]. The lack of blood flow creates a recalcitrant wound healing environment and the patients are at a higher risk for osteomyelitis. Often, patients will have both diabetes and PVD and have a 2- to 5.5-fold increase risk of ulceration leading to osteomyelitis [15, 59]. Patients with an impaired immune function may not have the ability to appropriately fight off an infection and thus are at a higher statistical risk of developing a deep bone infection. This includes patients taking corticosteroids for rheumatic or dermatologic diseases, patients receiving chemotherapy, organ transplant recipients, as well as systemic diseases like diabetes [25, 58, 60]. Uncontrolled diabetics live in a state of elevated glucose levels which impairs leukocyte function and can negatively affect the body’s ability to respond to antimicrobials [60].
The lower extremity itself is a risk factor for developing osteomyelitis and is well known to be a hard-to-treat anatomical location. The foot and ankle has a relatively thin soft tissue envelope covering deep anatomical structures. This makes the lower extremity highly susceptible to repetitive trauma especially in areas of boney prominences. Once bone is exposed, soft tissue coverage can be challenging. There are very limited options for local tissue coverage in the lower extremity. Surgeons have thus turned to free tissue transfer to increase soft tissue girth, but the complexity of these procedures can lead to significant complications in many patients, especially in the elderly or patients with diabetes, peripheral vascular disease, end stage renal disease, or infection [61, 62]. In addition, instability is often created when bone is resected from the foot or when partial amputations are performed. This creates a dysfunctional lower extremity and can also lead to other problems including new ulcerations. As mentioned previously, many patients with foot osteomyelitis have poor vascular supply and the inability to heal. Rather than undergoing numerous limb salvage procedures when osteomyelitis is involved, patients may be better served with a below-knee or above-knee amputation [61–66].
Diagnosis
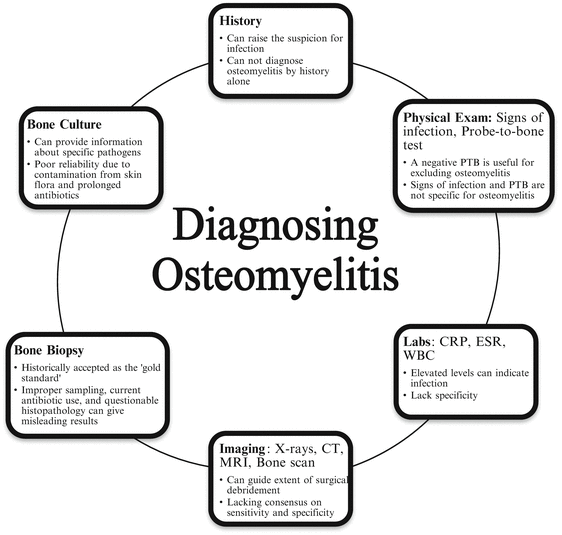
A unique challenge with osteomyelitis is definitively diagnosing the disease and making this diagnosis early. An accurate diagnosis is needed in order to formulate an appropriate treatment plan which is especially true for this progressive destructive process. There are several modalities used for identifying osteomyelitis including history, physical examination, laboratory values, imaging, microbiology, and bone biopsies (Fig. 1.1) [20, 67–81]. Ultimately, a combination of these modalities is needed to diagnose osteomyelitis. Each diagnostic modality has its own strengths and weaknesses with no single modality providing conclusive evidence of bone infection. To date, no single, robust, consensus-driven, diagnostic algorithm is available for clinicians to utilize for osteomyelitis. Since there is no standardized method available, ambiguous results and potentially failure of treatment can result.
An adequate history can be very informative for raising the suspicion and approaching a diagnosis of osteomyelitis. Frequent symptoms can include redness, swelling, pain, or drainage from a wound or surgical site. Often the pain is described as vague, deep, and chronic [4]. Any history of trauma or ulceration should be thoroughly investigated. Past medical history should be evaluated as well for systemic diseases and their current management and control. For example, it is important to evaluate glycemic control in diabetic patients. Other useful information includes nutritional status, ambulatory status, age, and presence of neuropathic or peripheral vascular symptoms [4, 26].
Physical examination and laboratory values for infection are two other commonly utilized modalities for the diagnosis of osteomyelitis. The physical signs of osteomyelitis are subjective in nature. This includes signs of infection of the overlying soft tissue envelope as well as the quality of the suspected area of bone infection. Fragmentation, necrosis, desiccation, and frank purulence of the bone are strong indicators of infection. However, these signs may not be specific for osteomyelitis. Fragmentation could be due to other factors including nutrition, age, Charcot neuro-osteoarthropathy, and trauma. Necrosis and desiccation could be the result of vascular compromise. Further, frank purulence may not be coming from the bone but from the surrounding soft tissue infection. The Grayson study recommended the “probe-to-bone” test for the diagnosis for osteomyelitis [19]. They reported a sensitivity of 66 %, specificity of 85 %, and a positive predictive value of 89 % with probe-to-bone test and presence of osteomyelitis. However, a subsequent study by Lavery et al. called into question the specificity for this test [68]. Their diagnosis had been confirmed with a bone culture and they found a sensitivity of 87 %, specificity of 91 %, positive predictive value of only 57 %, but a negative predictive value of 98 %. This shows that a negative probe-to-bone test may be more useful in excluding osteomyelitis than a positive test would be for confirming diagnosis. Elevated laboratory values including C-Reactive Protein, erythrocyte sedimentation rate, and white blood cell counts may be surrogate indicators of bone infection but lack specificity for osteomyelitis [82–84].
One of the major problems with diagnosing osteomyelitis is that imaging studies have low sensitivity to early detection and are non-specific. Plain radiographs, nuclear medicine studies, and magnetic resonance imaging are among the most commonly used imaging modalities for the diagnosis of osteomyelitis. Several studies have looked at the sensitivity and specificity of each without reaching a consensus on appropriate imaging [20, 67, 70–79, 85]. The second major issue is the difficulty in distinguishing between osteomyelitis and a different disease entity. This is especially troubling to the foot and ankle surgeon when dealing with diabetic patients. Sixty to seventy percent of diabetic patients have mild to moderate peripheral neuropathy and are at risk of developing neuro-osteoarthropathy [88, 89]. Charcot neuro-osteoarthropathy of the foot can often be mistaken for osteomyelitis both on physical examination as well as on imaging. Even more of a challenge is when both disease entities are present concomitantly.
Bone biopsy and bone culture are also commonly used to definitively diagnose osteomyelitis. In fact, it has long been purported that a bone biopsy is the gold standard for diagnosing osteomyelitis. However, it is not without its own challenges and problems due to improper sampling techniques, current use of antibiotics, or questionable histopathology results [80]. A study by Meyr et al. evaluated the reliability of histopathology of bone biopsies used for diagnosis of osteomyelitis in diabetic patients. They found a unanimous agreement between four board-certified pathologists for only 33.33 % of the specimens examined. Questionable results where at least one pathologist diagnosed “no evidence of OM” and at least one other pathologist diagnosed “findings consistent with OM,” occurred 41.03 % of the time [80]. Further, as discussed in a previous paragraph, osteomyelitis may be used as a descriptive histological term that may or may not indicate infection, rather than a diagnosis. Bone tissue cultures also pose an issue with specimen contamination and only specific bacteria being cultured [86, 87]. There is a risk of false-positive results from skin flora surrounding the bone, but also a risk of false-negative results due to prolonged release of antibiotics from bone [87]. Thus, there is poor reliability of bone cultures taken in the presence of a wound in determining the diagnosis of osteomyelitis as well as the infecting pathogen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree