Treatment
A wide array of analgesic strategies is currently available. Pain management approaches commonly used in advanced cancer are listed in
Table 69-3. Opioid-based pharmacotherapy is the preferred strategy for managing cancer pain. This approach has been generalized to the treatment of pain arising from other terminal conditions on the basis of theoretical rather than empiric grounds. The widespread success of this approach in controlling cancer pain has led to strong endorsement by numerous international and national organizations, as well as a federal task force (
44,
45,
46,
47). Countless chapters, books, and review articles published over the past decades have advocated the skillful integration of disease-modifying therapy with the use of opioid and nonopioid analgesics.
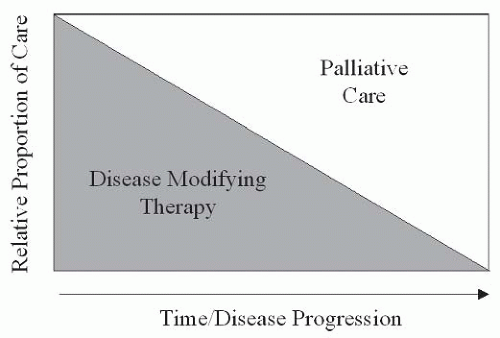
Disease-modifying therapy is the treatment of choice, irrespective of etiology, because it may permit definitive, lasting pain relief. For cancer patients, disease-modifying treatments are geared toward reduction or eradication of tumor. Therapies may include surgery, chemotherapy, radiation therapy, and the delivery of radiopharmaceuticals. The reduction of tumor effects on normal tissue may reduce or eliminate pain. Despite the widespread practice of delivering chemotherapy to address cancer pain, the analgesic benefits of this
approach remain unproven and pain alleviation in the absence of tumor shrinkage has been documented (
48,
49). Treatments targeting primary, noncancer, disease processes (e.g., antibiotics for infections) may offer effective pain control. Such interventions, offering emphatic symptom resolution, may obviate the need for further pharmacologic or interventional therapies.
Strong empirical data establish analgesic pharmacotherapy as the mainstay of cancer pain management (
40). Analgesics used for the management of pain associated with advanced disease are broadly grouped into three classes: nonopioid, adjuvant, and opioid. The WHO’s analgesic ladder for cancer pain management (
Fig. 69-2) advocates combining agents from these different classes in order to capitalize on their different mechanisms of action (
50). Clinical application of the WHO algorithm has been shown to control 80% to 90% of cancer pain although its superiority has yet to be established in a randomized, controlled clinical trial (
42,
51). The indications, benefits, and limitations of agents in each class are discussed as follows.
Aspirin and other salicylates, acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDs) constitute nonopioid analgesics. The WHO ladder advocates the use of nonopioids as first-line therapy for “mild” cancer pain. The safety and efficacy of NSAIDs as monotherapy in mitigating pain arising from many etiologies has been well documented (
52). Additionally, like acetaminophen, NSAIDs have opioidsparing effects that may avoid dose-limiting opioid side effects (
53). Bone pain from lytic metastases is, in part, prostaglandin mediated and therefore responsive to NSAID therapy (
54).
Unless contraindicated, patients should undergo serial nonopioid trials, particularly those with pain related to osseous metastases or inflammation. The maximal effective dose and degree of patient responsiveness varies considerably among NSAIDs (
39). Therefore, sequential trials may be needed to identify the optimal agent and dose. NSAID-related gastropathy and antiplatelet effects in thrombocytopenic patients are a significant concern. Adverse consequences may be reduced through the use of cyclooxygenase-2-selective inhibitors or gastroprotective agents (
55). Care must be taken in patients with advanced illness to monitor for hepato- and nephrotoxicity related to acetaminophen and NSAID use, respectively (
40).
Adjuvant analgesics, also referred to as
coanalgesics, comprise a large class of drugs with the capacity to offer pain relief in certain conditions. A majority of adjuvant analgesic trials have been conducted in patients with chronic neuropathic pain (e.g., postherpetic neuralgia). Their efficacy in this setting has served as justification for their use in pain arising from terminal disease states. The appropriateness of this strategy is attested by the inclusion of adjuvant analgesics as an integral part of all current guidelines (
50,
56,
57,
58). Adjuvant analgesics’ role as the preferred agents for control of neuropathic pain is supported by numerous clinical trials and literature reviews (
59,
60). The majority of adjuvant analgesics used to treat pain in advanced disease can be classed as antidepressants, anticonvulsants, or oral local anesthetics. However, the successful use of corticosteroids, antispasmodics, neuroleptics, and osteoclast inhibitors has been anecdotally described, and some agents have been found effective in controlled trials. The most frequently used agents are discussed below.
The tricylic antidepressants have been most extensively studied among the adjuvants and have well-documented efficacy (
61). Secondary amines (e.g., nortriptyline and desipramine) may offer less analgesia relative to tertiary amines (e.g., amitriptyline and imipramine), but have fewer associated anticholinergic side effects (
61,
62). Selective serotonin reuptake inhibitors have produced poor to mixed results in clinical trials. If cotreatment of depression is desired, the serotonin norepinephrine reuptake inhibitor duloxetine may be a superior choice, although its efficacy for cancer pain remains untested (
63).
Trials have established the anticonvulsant carbamazepine as a first-line agent for intermittent lancinating pain. However, the associated risk of causing leukopenia limits its use in patients at risk for hematological abnormalities (
64). Both gabapentin and pregabalin produce good results in the treatment of lancinating continuous neuropathic pain (
65,
66,
67,
68). Their benign side-effect profiles and the lack of drug-drug interactions make them reasonable first- or second-line choice for adjuvant analgesic therapy. Oxcarbazepine, levoteracitam, and lamotragine may be trialed on theoretical grounds as their utility in controlling pain associated with terminal illness lacks empirical support (
40).
Topical agents such as 2% to 4% viscous lidocaine may alleviate pain from mucosal lesions. The degree of analgesia afforded by dermally applied topical preparations is unclear. In a recent blinded, controlled trial, lidocaine-impregnated patches offered no greater relief than placebo for postincisional neuropathic pain in cancer patients (
69). A variety of topical salves combining various agents including amitriptyline, ketamine, gabapentin, etc. have been anecdotally endorsed, but empirical evidence is limited and their costs are often prohibitive (
70,
71).
In addition to NSAID therapy, several adjuvant analgesics are uniquely beneficial in the management of pain arising from bone metastases. These include steroids, bisphosphonates, and radiopharmaceuticals. Dexamethasone is the steroid of choice at doses ranging from 1 mg twice daily to 100 mg daily, with standard doses being 16 to 24 mg/d (
40,
72). Bisphosphonates inhibit osteoclast activity and effectively alleviate pain from bone lesions (
73). Single doses of the radiopharmaceuticals, strontium-89 and samarium, may lastingly alleviate pain arising from diffuse bone metastases as demonstrated in breast and prostate cancer cohorts (
74,
75).
An extensive international literature describes the control of cancer pain through the use of high-dose, long-term opioid therapy. Recommendations that doses be increased to “effect or side effect” stem from the lack of a therapeutic ceiling for opioid analgesia, although large dose increments may be required at high dose ranges to achieve incremental benefit. Many patients require doses as high as or higher than the equivalent of 5 g of oral morphine per day (
76,
77). Clinical success and an evergrowing literature has given rise to increased acceptance and comfort with high-dose opioid-based analgesia (
76,
77).
Opioids can be subclassified as pure agonists (e.g., morphine, oxycodone), agonist-antagonists (e.g., nalbuphine, pentazocine), and partial agonists (e.g., buprenorphine) based on their interaction with endogenous opioid receptors. With few exceptions, only pure µ agonists (e.g., morphine, methadone, oxycodone, hydromorphone, fentanyl) should be used to manage pain in patients with advanced diseases. Physiologic withdrawal syndromes may be precipitated by the use of ago-nist-antagonists in patients who are physically dependent on opioids (
78).
Administration of µ-receptor agonists has become increasingly refined over the past decades as new synthetic formulations and routes of administration have become available. Irrespective of these advances, general clinical practices have not been significantly altered (
79). Algorithms described in numerous guidelines, chapters, and review articles endorse the following steps:
Establish opioid analgesic requirements in “naïve” patients with liberal, as-needed dosing of an immediate-release (IR) (also referred to as normal-release) formulation.
Once patients’ use of the IR formulation has stabilized, generally after a 1 to 2 week interval, their total daily IR consumption is converted to an equivalent total daily dose of a sustained-release (SR) opioid preparation. When possible, the same IR and SR opioid should be used. Long-acting or SR formulations are generally IR opioids placed in time-release matrices so that they may be dosed at longer intervals. Methadone may be dosed on a schedule of every 8 or 12 hours and therefore may be included among the SR or long-acting opioids (
80).
Patients’ SR preparations should be supplemented with “rescue” or demand IR doses, typically 5% to 15% of the total daily dose, for preemptive use in anticipation of pain or when the SR preparation fails to provide adequate analgesia.
For example, a patient consuming an average of sixteen 10 mg IR oxycodone tablets per day would require twice daily 80 mg SR oxycodone tablets with rescue doses of 8 to 24 mg of IR oxycodone. Subsequent upward titration of the SR opioid to optimal effect is based on the frequency of IR rescue use. For patients with activity-related incident pain and minimal pain at rest (a scenario commonly encountered in patients with painful bone metastases), higher IR rescue doses and lower SR doses may provide superior analgesia and reduce cumulative daily opioid consumption.
The pharmacologic management of pain in patients with advanced disease requires flexibility in both the choice of medication and the dosing strategy. Contingency planning for rapid analgesic escalation should pain abruptly worsen is critical to long-term success. Oral medications offer both ease of administration and upward titration. At the time of the writing of this chapter, morphine and oxycodone are the only pure µ-agonists available in both IR and SR oral preparations. Methadone effectively alleviates cancer pain when dosed both “around-the-clock” and as an as-needed rescue medication (
81). However, methadone’s erratic pharmacokinetics demand
caution and use by experienced clinicians to avoid inadvertent, delayed toxicity (
82). Further, clinicians should be alert to the possibility of QT prolongation and torsades de pointes with daily methadone doses greater than 300 mg (
83,
84).
The transdermal route benefits patients lacking or expected to lose the capacity for enteral absorption. Fentanyl and buprenorphine patches are currently the only transdermal opioid formulations available in the United States. Data support the use of both transdermal fentanyl (
85,
86) and buprenorphine (
87,
88) to control cancer pain, despite the fact that beprenorphine is a partial µ-receptor agonist (
89). Fentanyl can also be administered transmucosally in the form of a lozenge that has rapid onset and a discrete duration of action making it a good choice for patients experiencing short intervals of severe pain with physical activity.
Parenteral opioid administration offers the capacity for rapid upward titration and accurate establishment of patients’ analgesic needs. Parenteral hydromorphone is a potent opioid (7.5 times more potent than parenteral morphine) that can be administered at concentrations of 20 mg/cc making subcutaneous administration feasible. Fentanyl, oxymorphone, methadone, and morphine are also available for parenteral administration. Morphines availability in long- and short-acting tablets, elixir (2, 4 mg/cc, 20 mg/mL), long-acting pellet (deliverable via nasogastric [NG] tube), and parenteral formulations, as well as highly concentrated subcutaneous preparations, allows tremendous dosing versatility. Also morphine is also quite inexpensive.
Opioid-related side effects need not limit upward dose titration (
90). Persistent somnolence can be managed with methylphenidate or modafinil after all nonessential centrally acting medications have been discontinued. A reasonable starting dose of methylphenidate is 2.5 mg twice daily (last dose provided no later than 2 p.m.). Typical effective daily doses range from 10 to 40 mg. Common side effects—including nausea, hyperhidrosis, xerostomia, and constipation, among others— can generally be effectively managed and need not limit opioid therapy (
90). When optimal opioid side-effect management proves ineffective, opioids should be rotated (
40).