25
Pain
1. Define the different types of pain.
2. Provide clinical implications for physical therapy regarding the different types of pain.
3. Describe the components and mechanisms of pain reception and transmission.
4. Explain the role of the sympathetic nervous system and substance P in pain reception and modulation.
5. Describe the theories associated with pain modulation and control.
6. Outline various clinical methods to measure and document pain perception.
7. Explain the role of physical agents in managing a patient’s pain.
8. Describe the purpose and components of a multidisciplinary treatment program for pain management.
Pain is an experience based on a complex interaction of physical and psychological processes. It has been defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.16,108,150 Pain usually acts as a warning to protect the body from damage and thus serves an essential function in survival.163 It is important to realize that pain is not just the activation of receptors of noxious stimuli, known as nociception, but also the sensory experiences, suffering, and alterations in behavior associated with such activation.
Pain is the most common symptom prompting patients to seek medical attention and is a predominant symptom leading patients to receive rehabilitation.162 Many patients with musculoskeletal or neurological impairments report pain, and most of these individuals consider pain control or relief to be the primary goal of treatment.72 Pain may alter body structure and function, limiting participation in home, work, and recreational activities. Pain symptoms encountered by rehabilitation professionals are generally related to inflammation of musculoskeletal or neurological structures caused by injury, trauma, or degenerative disease. These structures can be sources of pain and can increase the responsiveness of peripheral pain receptors to other painful stimuli.49,141,145
TYPES OF PAIN
Pain can be categorized according to its duration or source as acute, chronic, or referred. Acute pain is generally defined as pain of less than 6 months duration for which an underlying pathology can be identified.17 Acute pain is felt in response to actual or potential tissue damage that resolves when tissue damage or the threat of damage passes. Chronic pain persists beyond the normal time for tissue healing.49 Chronic pain conditions are usually the result of activation of dysfunctional neurologic or psychological responses that cause the individual to continue to experience the sensation of pain even when no damaging or threatening stimulus is present. Referred pain is the experience of pain in one area when the actual or potential tissue damage is in another area. Knowing whether a patient’s pain is acute, chronic, or referred will help the clinician determine the mechanisms and processes that may be contributing to the sensation and facilitate selection of the most appropriate intervention to control or relieve this symptom.
Acute Pain
Acute pain is a complex combination of unpleasant sensory, perceptual, and emotional experiences with associated autonomic, psychological, emotional, and behavioral reactions that occur in response to a noxious stimulus provoked by acute injury or disease.15,161 Acute pain is generally viewed as biologically meaningful, useful, and time-limited. Acute pain is mediated through rapidly conducting pathways and is associated with increases in muscle tone, heart rate, blood pressure, skin conductance, and other manifestations of increased sympathetic nervous system activity.106 The intensity and location of the pain are usually related to the degree of tissue inflammation, damage, or destruction in the area in which the pain is felt. Acute pain is generally well-localized and defined, although its degree of localization varies to some extent with the type of tissue involved. Acute pain lasts as long as the noxious stimulation persists. Acute pain serves a protective function after an injury by limiting activity to prevent further damage and promote tissue healing and recovery; however, it may also adversely affect an individual’s quality of life and impair the ability to function. For example, when a patient sustains a rotator cuff injury while playing racquetball, he or she will feel acute pain in the shoulder. This pain will probably cause the patient to restrict shoulder motion and thereby avoid further injury. This acute pain will likely prevent the patient from playing racquetball for a number of days or weeks but will gradually resolve as the injured tissues heal, allowing return to full activity.
Chronic Pain
Chronic pain may start as acute pain related to a chronic disease as with peripheral polyneuropathy, poststroke, or spinal cord injury pain syndrome, and fibromyalgia, or it may have no identifiable cause. Chronic pain is usually defined as pain that does not resolve in the usual time it takes for the disorder to heal or that continues beyond the duration of noxious stimulation.13 Some authors and organizations use time-based definitions, defining chronic pain as any pain lasting longer than 3 months or 6 months.28,63 Whatever its precise definition, chronic pain is an ongoing condition that is difficult to manage. It has been estimated that approximately one third of the United States population has chronic pain, and 14% of the U.S. population suffers from chronic pain related to the joints and the musculoskeletal system.95,117 Other disease states, such as cancer, are also commonly associated with chronic pain. A study of pain in 13,625 elderly and minority nursing home residents with cancer found that more than 25% reported daily pain.9
Chronic pain may be classified according to pathophysiology.1,36,42 Nociceptive pain is caused by the stimulation of pain receptors by noxious mechanical, chemical, or thermal stimuli and associated with ongoing tissue damage. Conditions associated with chronic nociceptive pain include arthritis, ischemia, cancer, and chronic pancreatitis. Neuropathic pain is the result of peripheral or CNS dysfunction without ongoing tissue damage. Neuropathic pain is seen in diabetic neuropathy, postherpetic neuralgia, and phantom limb pain. Mixed pain syndromes are those with multiple or uncertain pathophysiology. Examples include recurrent headache and some vasculitic syndromes. Psychological pain syndromes are those in which psychological processes play a large role. This kind of pain may be seen in somatoform disorders and conversion reactions. Although this classification of pain can be helpful for systematically approaching a patient with pain, the pathophysiology of chronic pain in an individual patient is often only partially understood. In addition, chronic pain may have more than one cause. The pain associated with arthritis, for example, may be caused by inflammation, joint distortion, strain on muscles and connective tissue, and microfracture from eroded cartilage or bone. This pain may also be magnified by psychological distress related to loss of function.36
Chronic pain may be the result of changes in sympathetic nervous system and adrenal activity, reduced production of endogenous opioids, or sensitization of primary afferent (peripheral sensitization) and spinal cord neurons. Decreased levels of enkephalins and increased numbers and sensitivity of nociceptors have been observed in individuals with chronic pain.87 These individuals frequently have increased sensitivity to both noxious (hyperalgesia) and innocuous (allodynia) stimuli.113 These changes in pain perception may in part be the result of a process known as wind-up, or central sensitization, in which the pathways that transmit pain continue to discharge after the discontinuation of intense or repeated stimulation. Then, even a small additional stimulus exceeds the threshold that is perceived as painful.29,30,122,178 Thus, for an individual with a painful condition that is severe or long-lasting, the noxious stimulation may result in increased pain receptor activity and a consequent reduced tolerance for noxious or innocuous stimuli. Understanding of this sensitization mechanism has led to increased study and use of preemptive analgesia before procedures that are known to be painful in an attempt to reduce postprocedural pain and reduce recovery time.22,47,68
Although many patients with chronic pain have found ways to adapt and cope with their condition, there are some who catastrophize or perceive their symptoms to be debilitating. These patients are more likely to have difficulties with social function and employment. Some individuals, such as those with preexisting affective or anxiety disorders, or chemical dependence, are predisposed to becoming more dysfunctional in the face of chronic pain. Psychological and social factors associated with chronic pain include depression; catastrophizing; decreased function, quality of life, and ability to work; and increased dependence on others. In a study of 5,800 patients, 41% of those with depression reported disabling chronic pain compared with 10% of those without depression.2 Patients with concurrent depression and disabling chronic pain had significantly poorer health-related quality of life, greater somatic symptom severity, and higher prevalence of panic disorder than other patients.2 A study of 1800 depressed older adults also found that patients with coexisting arthritis (56%) who were treated for depression not only had decreased depressive symptoms but also had reduced pain and improved function and quality of life.92 Catastrophizing has been positively correlated with the reported severity of pain, affective distress, muscle and joint tenderness, pain-related disability, poor outcomes of pain treatment, and possibly inflammatory disease activity.32 These associations exist even after controlling for depression. Proposed effects of catastrophizing range from maladaptive influences on the social environment to direct amplification of the processing of pain by the CNS.32 Although most studies have found a correlation between chronic pain and psychological factors, no studies have confirmed this relationship, making this an area in need of further research.71
Chronic pain can decrease a person’s participation in normal activities. A study in Europe found that more than half of a group of patients with chronic pain were less able or unable to work outside the home, 19% had lost their jobs, and 13% had changed jobs because of their pain.19 Chronic pain also impacts a person’s relationship to others, particularly those in the caregiving role. In an enmeshed or solicitous relationship, the patient assumes a sick role that is unknowingly reinforced by the caregiver through “checking” behaviors and excessively supportive responses. It is important to note that although health care professionals may suspect that a patient is using chronic pain for secondary gain, to play the sick role, or to justify certain behaviors, this attitude may obstruct a patient’s adjustment to chronic pain, prolong sick leave, and hinder rehabilitation.52
Ideally, the development of chronic pain should be prevented by early identification of individuals at risk. Patients with prolonged, severe, or disabling acute pain are at increased risk of developing chronic pain. To reduce this risk, pain-controlling interventions, such as physical agents or medications, should be applied during the acute stage of an injury and during the later recovery phases, when pain is still the result of pain receptor activation.14 The prevention of chronic pain in patients who have had surgery should include avoiding nerve damage while in surgery and effective pain control immediately after surgery, because the intensity of acute postoperative pain correlates with the risk of developing chronic pain.78
If chronic pain develops, successful treatment usually requires that all components of the dysfunction be addressed. Multidisciplinary treatment programs based on a biopsychosocial model of pain have been specifically developed to address these problems.163 These treatment programs are described in the section on pain management.
Referred Pain
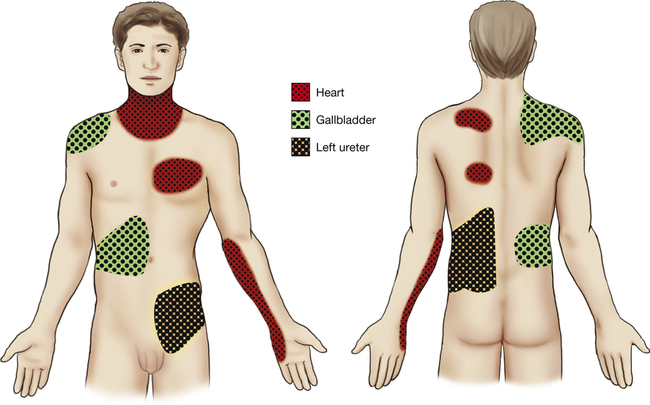
Referred pain is felt at a location distant from its source. Pain may be referred from one joint to another, from a peripheral nerve to a distal area of innervation, or from an internal organ to an area of musculoskeletal tissue (Fig. 25-1). For example, hip joint pathology occasionally refers pain to the knee, particularly in children, and compression of the spinal nerve roots at the L5 to S1 level as they exit through the spinal foramen may cause pain in the lateral leg because this is the area of sensory innervation.81,153 Common referral patterns from internal organs to musculoskeletal tissue include the pain associated with myocardial infarction or angina caused by cardiac ischemia that is felt in the upper chest, left shoulder, jaw, and arm, and pain originating from the central portion of the diaphragm that is frequently felt in the lateral tip of either shoulder. The gallbladder also frequently refers pain to the right shoulder or the inferior angle of the right scapula, and the spleen refers pain to the left shoulder.
It is proposed that pain is referred in one of three ways: from a nerve to its area of innervation, from one area to another derived from the same dermatome, or from one area to another derived from the same embryonic segment. The peripheral neural pathways from these different areas converge on the same or similar areas of the spinal cord and synapse with the same second-order neurons to ascend the spinal cord and reach the central cortex.175 For example, pain is referred from the diaphragm to the tip of the shoulder because both of these areas initially develop in the neck region during embryonic development, causing them both to have efferent innervation from the phrenic nerve and afferent innervation to the second through fourth levels of the cervical spine. When pain that may be of either visceral or musculoskeletal origin converges on the same neuron in the spinal cord, it is usually interpreted to be of musculoskeletal origin. This may be because musculoskeletal injury and pain are so much more common that the brain “learns” that activity arriving along that pathway is associated with pain stimulus in a particular musculoskeletal area.
MECHANISMS OF PAIN RECEPTION AND TRANSMISSION
Specific nerve endings called nociceptors respond to all painful stimuli, and specific nerve types, small myelinated A-delta fibers and unmyelinated C fibers, transmit the sensation of pain from these nerve endings to the spinal cord and then, within specific tracts, to the brain. The quality of the pain depends on the type of tissue from which the stimulus originates and which of the two nerve types transmits the pain; the intensity of the pain is related to the firing rate of the nerves. Pain from cutaneous noxious stimulation is usually perceived as sharp, pricking, or tingling and is easy to localize, whereas pain from musculoskeletal structures is usually dull, heavy, or aching and is harder to localize.155 Visceral pain has an aching quality similar to that of musculoskeletal pain but tends to refer superficially rather than deeply.80
Pain transmitted by C fibers is usually dull, long-lasting, and aching, whereas pain transmitted by A-delta fibers is generally sharp. The intensity of the pain and the responses to it are thought to be more severe when the intensity of nociceptive receptor stimulation is greater than that of nonnociceptive receptor stimulation, when levels of endogenous opioids are low, and with certain variations in the individual’s psychological state.182
Pain Receptors
Nociceptors are free, noncorpuscular peripheral nerve endings consisting of a series of spindle-shaped, thick segments linked by thin segments to produce a “string-of-beads” appearance. The beads and end bulbs contain mitochondria, glycogen particles, vesicles, and bare areas of axolemma that are not covered by Schwann cell processes.59 Nociceptors are present in almost all types of tissue. For example, low back pain is thought to be transmitted from free nerve endings that have been found in facet joints, discs, ligaments, nerve roots, and muscles.23 In damaged discs, nerves also penetrate areas where they would normally not be present, such as the inner annulus fibrosus and the nucleus pulposus, thus contributing to discogenic pain.119
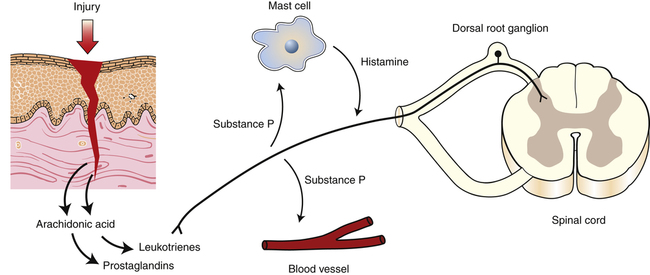
Nociceptors can be activated by intense thermal, mechanical, or chemical stimuli from exogenous or endogenous sources. For example, intense mechanical stimulation, such as that caused by a brick falling on someone’s foot or a piece of broken bone compressing a nociceptor, will result in nociceptor activation. Chemical stimulation by exogenous substances, such as acid or bleach, or by endogenously produced substances, such as bradykinin, histamine, and arachidonic acid, which are released as part of the inflammatory response to tissue damage, can also activate nociceptors. Because these chemical mediators remain after the initial physical stimulus has passed, they generally cause pain to persist beyond the duration of the initial noxious stimulation. It is important to note that chemical mediators of inflammation also sensitize nociceptors, reducing their activation threshold to other stimuli.5,8 This is the reason that clinically many activities and stimuli to recently injured areas are perceived as painful even when they are not damaging.
When nociceptors are activated, they release a variety of neuropeptides from their peripheral terminals, including substance P and a number of breakdown products of arachidonic acid, such as prostaglandins and leukotrienes.87 Nociceptors also convert the initial stimulus into electrical activity, in the form of action potentials, by a process known as transduction (Fig. 25-2). It is thought that the released neuropeptides may initiate or participate in transduction because they sensitize nociceptors.174 The action potentials resulting from the process of transduction propagate from the nociceptors along afferent nerves toward the spinal cord.
Peripheral Nerve Pathways
Nociceptors give rise to two types of first-order afferent nerve fibers, C fibers and A-delta fibers. The activity in both types of fibers increases in response to peripheral noxious stimulation, including that associated with acute inflammation or muscle ischemia.∗ Eighty percent of afferent pain-transmitting fibers are C fibers, and the remaining 20% are A-delta fibers.38 Generally, about 50% of the sensory fibers in a cutaneous nerve have nociceptive functions.182
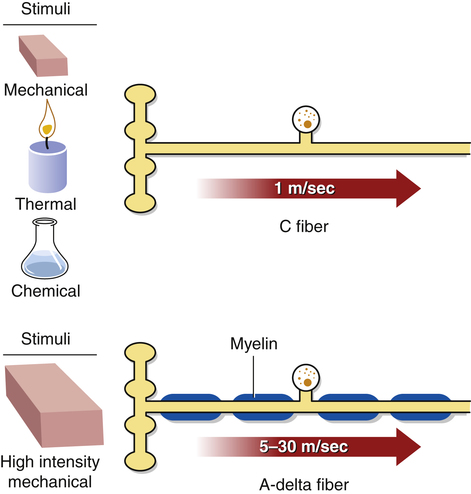
C fibers, also known as group IV afferents, are small, unmyelinated nerve fibers that transmit action potentials quite slowly, at the rate of 1.0 to 4.0 m/sec.33 They respond to noxious levels of mechanical, thermal, and chemical stimulation, causing pain that is generally described as dull, throbbing, aching, or burning and may also be reported as tingling or tapping (Fig. 25-3).115,154 The pain sensations transmitted by these fibers have a slow onset after the initial painful stimulus, are long-lasting, emotionally difficult for the individual to tolerate, and tend to be diffusely localized, particularly when the stimulus is intense.54,97 They can be accompanied by autonomic responses such as sweating, increased heart rate and blood pressure, or nausea.177 The pain associated with C fiber activation can be reduced by opiates, and this pain relief is blocked by the opiate antagonist naloxone.167
A-delta fibers, also known as group III afferents, are also small-diameter fibers; however, they transmit more rapidly than C fibers, at a rate of about 30 m/sec, because they are myelinated.33,58 They are most sensitive to high-intensity mechanical stimulation, although they can also respond to stimulation by heat or cold and are capable of transmitting innocuous information.114 The sensations associated with A-delta fiber activity are generally described as sharp, stabbing, or pricking.182 The pain sensations transmitted by these fibers have a quick onset after the painful stimulus, last only for a short time, are generally localized to the area from which the stimulus arose, and are not generally associated with emotional involvement. The pain associated with A-delta fiber activation is generally not blocked by opiates.48
A-beta nerve fibers can also be involved in abnormal pain transmission and perception. A-beta fibers, or wide dynamic-range neurons, have relatively large myelinated axons that conduct impulses more quickly than A-delta and C fibers. Their receptors are located in the skin, bones, and joints. Normally, they transmit sensation related to vibration, stretching of skin, and mechanoreception and do not transmit pain. However, in states such as neuropathic pain and central sensitization, these neurons alter their transduction so that normal stimuli result in pain. There are three theories on how A-beta fibers contribute to pain.132 The first theory states that firing of A-beta fibers activates spinal neurons that have undergone central sensitization. The second theory posits that A-beta fibers might sprout into spinal cord layers normally targeted by C-fibers and when activated, stimulate the wrong neurons.179 A third theory states that intact A-beta nerve fibers near damaged nociceptive nerves begin firing abnormally.180 This alteration in nerve function is key in prolonged pain.
Central Pathways
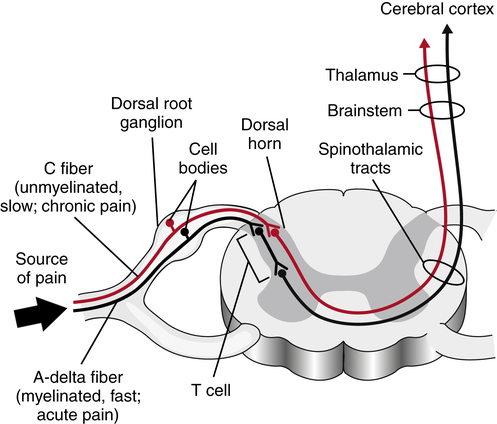
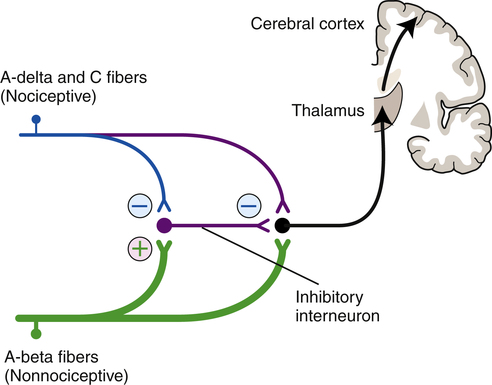
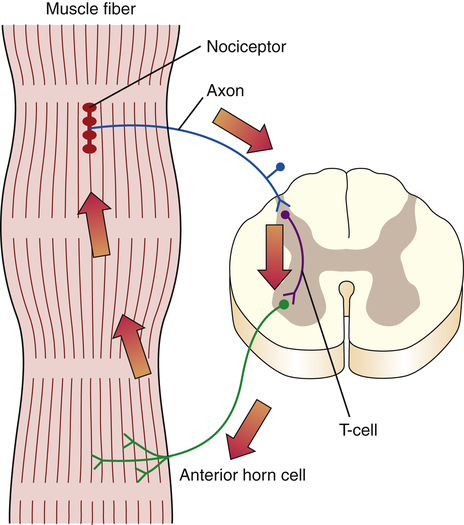
The peripheral first-order C and A-delta afferents project from the periphery to the grey matter of the spinal cord. The C and A-delta fibers synapse, either directly or via interneurons, with second-order neurons in the superficial dorsal horn of the gray matter (the substantia gelatinosa).33,85,89–91 Some A-delta fibers penetrate more deeply into the dorsal horn to terminate at the normal termination sites of A-beta afferents. The interneurons in the dorsal horn are also known as transmission cells, or T cells (Fig. 25-4).
T cells make local connections within the spinal cord, either with efferent neurons as part of spinal cord reflexes or with afferent neurons that project toward the cortex. Continued or repetitive C-fiber activation can sensitize the T cells, causing them to fire more rapidly and to increase their receptor field size, and input from other interneurons originating in the substantia gelatinosa of the spinal cord or from descending fibers originating in higher brain centers can inhibit T-cell activity.10 Inhibitory interneurons in the substantia gelatinosa are activated by input from large-diameter, myelinated, low-threshold sensory neurons (primarily A-beta nerves) that respond to nonpainful stimuli.60,103 These inhibitory interneurons release various neurotransmitters, including norepinephrine, serotonin, and enkephalins, to modulate the flow of the afferent pain pathways.6,39,157 Thus the transmission cells receive excitatory input from the C fibers and A-delta nociceptor afferents and inhibitory input from large-diameter, nonnociceptor sensory afferents and from descending fibers from higher brain centers (Fig. 25-5).182
The balance of these excitatory and inhibitory inputs influences whether the individual feels pain and how severe the pain sensation is.103 The inhibition of pain by inputs from nonnociceptor afferents is known as pain gating and is discussed in greater detail in the section on pain modulation and control theories.
Transmission cell activation can increase muscle spasm via a spinal cord reflex in which the transmission cell synapses with anterior horn cells to cause muscle contractions. The ongoing muscle contractions can cause accumulation of fluid and tissue irritants. The contracting muscles may also initiate further nociceptive impulses by mechanically compressing the nociceptors. In this way the combination of ongoing chemical and mechanical stimuli can set up a self-sustaining cycle of pain causing muscle spasm, which then causes more pain. This is known as the pain-spasm-pain cycle (Fig. 25-6). Many interventions indirectly reduce pain, even after their direct analgesic effect has passed, because they reduce muscle spasms and thereby interfere with this self-perpetuating cycle.
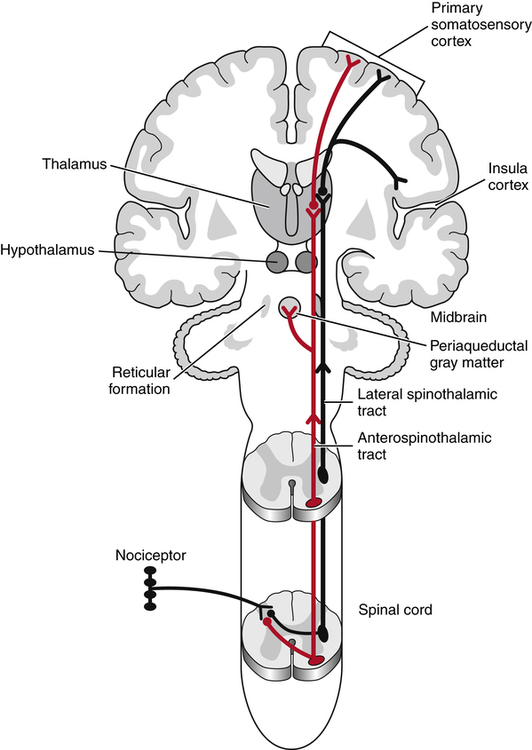
Ascending second-order nerves carry stimuli within the spinal cord toward the higher brain centers (Fig. 25-7). The second-order pathways that carry pain stimuli are located primarily in the anterolateral aspect of the cord.173 This area of the spinal cord also transmits information about temperature and touch. Most axons in the anterolateral system cross midline in the spinal cord to ascend contralaterally. Information regarding pain is transmitted within the anterolateral cord via both the lateral spinothalamic tract and the larger anterospinothalamic tract to project to the thalamus. The lateral spinothalamic tract projects directly to the medial area of the thalamus, whereas the anterospinothalamic tract separates from the lateral spinothalamic tract in the brain stem to synapse with neurons in the reticular formation and the hypothalamic and limbic systems, to then project to lateral, ventral, and caudal areas of the thalamus. The anterospinothalamic tract also relays information to the periaqueductal gray matter, which has a large concentration of opiate receptors and is thought to be associated with pain modulation. Impulses relayed via the lateral spinothalamic tract are involved in transmission of sharp pain and in localization of the painful stimulus, whereas those sent via the anterospinothalamic tract are involved in transmission of more prolonged, aching pain and are thought to have stronger association with the disturbing emotions that accompany the pain sensation. The second-order neurons synapse in the thalamus with third-order neurons to project to the cortex, from which the sensation of pain can reach consciousness.
Sympathetic Nervous System Influences
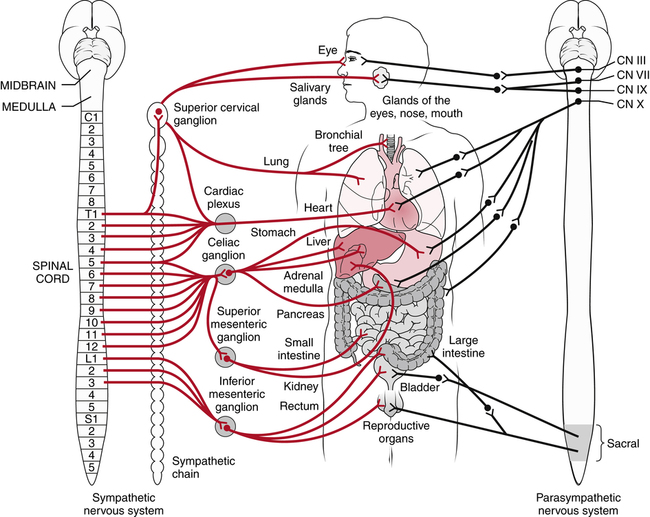
The sympathetic nervous system is a component of the autonomic nervous system. The autonomic nervous system consists of the sympathetic and parasympathetic systems and is concerned with the activities of smooth and cardiac muscles and with glandular secretion. This contrasts with most of the nervous system, which is concerned with voluntary activation of the skeletal muscles or with transmission of sensory impulses from the periphery (Fig. 25-8).45,64 The sympathetic nervous system is considered to be primarily involved in producing effects that prepare the body for “fight or flight,” such as increasing heart rate and blood pressure, constricting cutaneous blood vessels, and increasing sweating in the palms of the hands. Although it is normal for the sympathetic nervous system to be activated by acute pain or injury, stimulation of the sympathetic nervous system efferents does not usually cause pain.65 However, abnormal sympathetic activation, caused by a hyperactive response of the sympathetic nervous system to an acute injury or by a failure of its response to subside, can increase pain severity and cause exaggerated signs and symptoms of sympathetic activity, such as excessive vasomotor or sweating reactions. In patients with these signs and symptoms, pain relief can often be achieved by interrupting sympathetic nervous system activity by chemical or surgical means.18,21,84 In addition, stimuli that evoke sympathetic discharges, such as the startle reflex or emotional events, frequently exacerbate pain. It has therefore been proposed that excessive sympathetic nervous system activation may increase or maintain pain.45,64 Although anesthetic blockade of the sympathetic nervous system is widely used to reduce pain in complex regional pain syndrome (CRPS), its effectiveness has not yet been proven.24,25
Pain that is believed to involve sympathetic nervous system overactivation has a variety of names, including causalgia, reflex sympathetic dystrophy (RSD), shoulder-hand syndrome, posttraumatic dystrophy, Sudeck atrophy, and sympathetically maintained pain.123 Currently, the International Association for the Study of Pain (IASP) recommends the use of the term complex regional pain syndrome.90 CRPS involving tissue damage without nerve damage is categorized as type I, and CRPS associated with nerve involvement is categorized as type II.90 In addition, pain that is reduced with sympathetic blockade may be called sympathetically maintained pain (SMP), whereas pain that does not respond to sympathetic blockade may be called sympathetically independent pain (SIP).90
CRPS generally includes the following signs and symptoms: severe pain that is out of proportion to the inciting injury or disease, hyperesthesia (excessive reaction to painful stimuli), and allodynia (the sensation of pain in response to stimuli that are not usually painful). CRPS frequently also includes trophic changes such as skin atrophy and hyperhidrosis, edema, stiffness, increased sweating, and decreased hair growth.90 These symptoms generally result in decreased function and if the syndrome is prolonged, spotty osteoporosis in the affected area.37 Other sensory, vasomotor, and skeletal motor abnormalities have also been associated with this syndrome.135 CRPS can occur in any area of the body but is most common in the hand and in such cases, is frequently associated with ipsilateral restriction of shoulder motion. CRPS may develop as a consequence of major or minor trauma, after visceral disease or CNS lesions, or without any known antecedent event.
The Role of Substance P
Substance P is a neurotransmitter thought to be involved in the transmission of neuropathic and inflammatory pain. Substance P is present in both the central and peripheral nervous systems. In the CNS, it is found in approximately 20% of C fibers.168 It is also released from peripheral nociceptors and has been detected in inflammatory exudate.41,86,98 Substance P release can excite pain-transmitting neurons in the dorsal horn of the spinal cord and is involved in nociceptive processing at the spinal cord level.∗
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree