Chapter 46 Outcome Measures in Surgery of the Elbow
Introduction
Assessment of the outcome of patient treatment is becoming increasingly important, with service commissioners considering reported outcomes when selecting contracts.1 There is an array of purpose-developed, validated assessment tools that are region-, joint- or disease-specific to allow a treating surgeon to select an appropriate measure. This chapter will outline the rationale behind outcome assessment tools, the steps undertaken in developing and validating an outcome tool (with reference to the Oxford Elbow Score) and then summarize the assessment tools in current usage.2,3
Background
Clinical outcome assessment tools
The need to measure the outcome of treatment has long been recognized.4 Relying on a patient’s comments to the treating clinician is an unreliable way of gaining an objective insight into the results of treatment. Purpose-developed assessment tools have been designed to give a better picture of a patient’s status. These tools may be generic, or specific to a particular anatomical region or disease process.5–12 The tools will generally consider a number of domains (subsections) in order to obtain information about different issues that might be relevant to the outcome or a patient’s status. There is a balance to be struck between a tool that covers many domains, and so gives a comprehensive assessment of a patient’s status, and one that is sufficiently concise to be of practical use (i.e. can be completed accurately in a reasonable time).
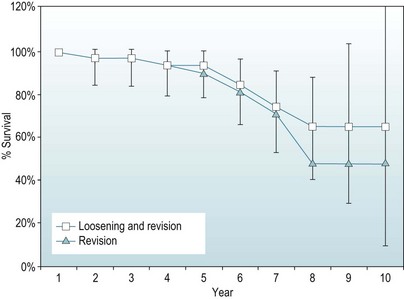
Survival analysis
A second technique involves determining a life table, considering all events that occur in a given year of follow-up together, and so recalculating the proportion surviving only once per given year of follow-up. This gives a simpler to follow, although annually averaged, picture of survival. An example is given in Figure 46.1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree