Fig. 8.1
Walch classification of glenoid morphology. A1 Centered humeral head, A2 central glenoid erosion, B1 posterior subluxation of humeral head, B2 posterior glenoid erosion, C dysplastic glenoid (>25° retroversion) (From Walch et al. [15])
The loss of the normal lubricating and load-bearing properties of the cartilage produces abnormal loads on the subchondral bone plate. The bone responds by remodeling, producing the sclerotic appearance on radiographs.
Synovial fluid can pass through microfractures of the subchondral bone plate, creating subchondral cysts (Fig. 8.2 ). Osteophytes form at the articular margins. Late-stage OA can develop osteonecrosis, leading to collapse of the humeral head.
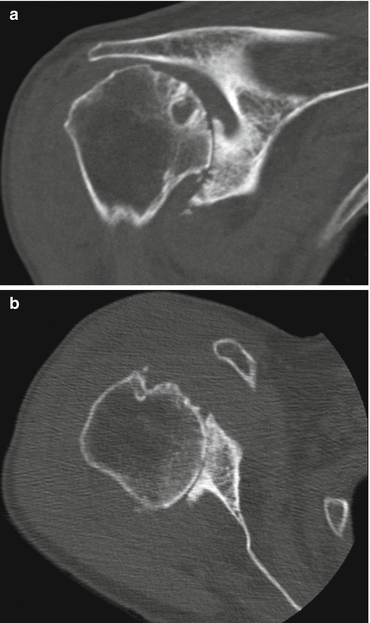

Fig. 8.2
Computed tomography images of rotator cuff tear arthropathy of the shoulder. Multiple factors contributing to joint stiffness are present: complete loss of joint space (a, b), collapse of subchondral bone (a), osteophyte formation (b), and subluxation of the humeral head in both superior (a) and posterior (b) directions (Copyright GI Bain. Used with permission)
A block to movement of the glenohumeral joint can arise from the increased retroversion of the joint (glenoid wear), flattening and incongruity of the humeral head (Fig. 8.3 ), and impingement against osteophytes.
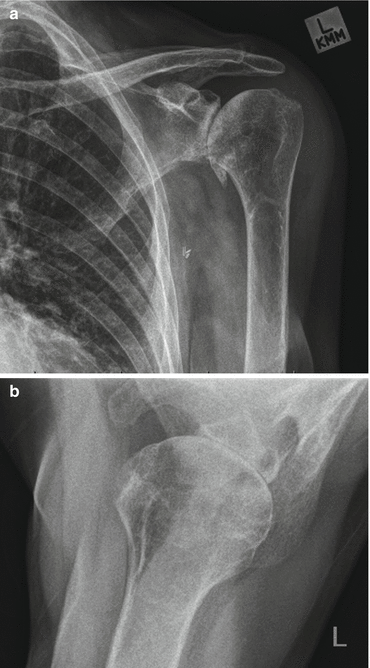

Fig. 8.3
Plain radiographs of osteoarthritic shoulder. Note multiple factors contributing to shoulder stiffness. (a) Osteophyte formation around inferior border of humeral articular surface. (b) Loss of joint space, erosion of posterior glenoid, posterior subluxation of humeral head on glenoid face (Copyright GI Bain. Used with permission)
Secondary Contracture
Wear of the posterior aspect of the glenoid sets up an environment for progressive contracture of the anterior soft tissues. The posterior capsule is stretched over the posterior osteophytes and by the internally rotated and posteriorly translated position of the humeral head [16]. The stretched capsule allows further posterior subluxation and internal rotation.
On the anterior side, the reverse occurs: the lack of external rotation of the humeral head decreases the resting tension in the anterior capsule and contracture develops in the anterior ligamentous structures (capsule and coracoclavicular ligament) [17]. The subscapularis muscle displays both shortening of the muscle tendon unit and adhesions to the surrounding structures [18]. Three distinct zones of the subscapularis tendon have been described: the superior tubular tendon (STT), the middle tendon, and, inferiorly, the muscle fibers that insert directly onto the humerus [17].
8.2.2 Inflammatory Arthropathy
8.2.2.1 Etiology
A large number of conditions may cause inflammation of the synovial lining of the joint capsule. These may be autoimmune syndromes such as rheumatoid arthritis and the seronegative arthropathies or crystal arthropathy syndromes such as gout.
8.2.2.2 Pathology
The specific effects vary according to the different etiologies, but in all cases the chronic inflammatory processes result in cytokine release and macrophage activation. This results in destruction of both cartilage and soft tissue.
In rheumatoid and seronegative inflammatory conditions, the changes are typically present throughout the entire joint, compared to the relatively focal nature of joint changes in osteoarthritis.
The disease process affects multiple joints. The presence of cervical spine instability due to inflammatory arthritis will limit the patient’s ability to undergo surgery. Multiple other body systems may also be affected, including the lungs, cardiovascular system, and skin.
8.2.2.3 Secondary Contracture
The damage to the capsular and surrounding tissues can affect the fulcrum function of the joint, resulting in reduced active and passive motion. Severe capsular attenuation and disruption of the rotator cuff tendons can result in loss of normal constraint, with subluxation of the humeral head on the glenoid. Persistent inflammation will not only destroy the articular cartilage but can also precipitate collapse of the subchondral bone, resulting in a loss of articular congruency.
8.2.3 Management
8.2.3.1 Clinical Presentation
The patient has pain with activity and potentially at rest. In inflammatory arthritis the pain is classically noticeable in the morning, whereas in osteoarthritis the pain becomes more of a problem as the day progresses. There is reduced active and passive glenohumeral range of motion in all movements.
In osteoarthritis, there is typically contracture of the anterior and inferior soft tissues resulting in a block to both abduction and external rotation. In severe cases, the patient may develop a fixed internal rotation contracture.
In inflammatory arthropathy, there may be other body systems affected and these must all be assessed when determining the patient’s suitability for treatment. Skin lesions present an infection risk, especially if arthroplasty is planned. These may be a result of the inflammatory disease process (e.g., psoriasis) or a result of underlying joint deformity (foot ulcers in rheumatoid arthritis). Specific attention must also be paid to the presence of any cervical spine instability.
8.2.3.2 Investigation
Plain radiographs demonstrate the key findings of reduced joint space, osteophytes, subchondral sclerosis, and subchondral cysts. An axillary lateral view will demonstrate the glenoid version (Fig. 8.2 ). If there is any concern regarding the glenoid morphology or bone stock, a CT scan is required to further quantify this.
8.2.3.3 Treatment
Various nonoperative treatments have been described for shoulder OA including physical therapy, oral cartilage supplements, and injections of either steroid or viscosupplements. There is little published evidence on the effectiveness of these modalities in managing the established secondary soft-tissue contracture.
Medical management of inflammatory arthritis can prevent joint destruction, but cannot reverse changes that have already occurred.
Surgical options for glenohumeral arthritis include arthroscopic debridement with capsular release, open debridement procedures, arthroplasty procedures, and arthrodesis.
Arthroscopic surgery for arthritis involves debridement of unstable cartilage fragments, removal of loose bodies, and excision of impinging osteophytes. A capsular release can also be performed and in most cases of OA this would involve the anterior and inferior capsule. Any adhesions around the subscapularis muscle can also be released, and a partial release of the tendon can also be performed.
The majority of patients with symptomatic shoulder arthropathy require an arthroplasty procedure. Concerns regarding progressive lucent lines around glenoid components [19] have led some surgeons to favor hemiarthroplasty procedures, especially in younger patients. However, the results of hemiarthroplasty, with or without glenoid reaming [19, 20] or with soft-tissue interposition arthroplasty [21], are generally inferior to those of total shoulder arthroplasty (TSA).
Total arthroplasty prostheses are either an anatomic design or a reverse prosthesis. Anatomic TSA requires strict rebalancing of the rotator cuff and capsular tissues. This can be achieved with a combination of capsular release, posterior capsular plication, and alteration of the humeral version [22].
The subscapularis muscle can also be addressed by release of any surrounding adhesions and lengthening procedures. Lengthening can be achieved by release of the superior tubular tendon component [17] or repair of the tenotomized tendon to a lateral stump of capsule [16, 18].
Reverse TSA has traditionally been indicated for patients with rotator cuff insufficiency. The component design has greater containment than anatomic TSA, allowing a greater margin for soft-tissue release without compromising stability. This is also an advantage in inflammatory arthroplasty where the soft tissues may be compromised. The uncemented glenoid component of a reverse TSR allows combined bone grafting procedures to address excessive retroversion [1, 22, 23]. Conversely, uncemented glenoid components have shown poor results in anatomic TSA [1–3, 24] .
8.2.4 Outcomes
Arthroscopic procedures are potentially attractive for addressing capsular contracture, especially in the setting of lower-grade articular cartilage changes. However, literature on these techniques is limited to case series. A recent review [4, 25] could not find any high-level evidence to support the use of arthroscopic debridement in the management of shoulder OA.
Anatomic TSA has demonstrated good to excellent functional scores [2, 26] and 90–95 % survival at 10 years [2, 22]. A recent systematic review [1, 27] reported that complications occur in 23 % of unconstrained shoulder arthroplasty cases. However, 20 % of these complications were related to hemiarthroplasty procedures [3, 4, 27]. The most common complication in anatomic TSA is glenoid loosening, although this may be present on radiographs but clinically silent [4, 27–29]. Gonzalez et al. [5, 27] reported 29 % of patients with glenoid loosening required revision.
Failure to correct glenoid retroversion, or to rebalance and stabilize the soft tissues can result in higher rates of instability and prosthesis failure [5, 22]. An intact rotator cuff is also necessary for the normal functioning of an anatomic TSA and rotator cuff insufficiency is a common cause for instability of the prosthesis [3, 30].
Reverse TSA has been reported to have a high complication rate. A meta-analysis of 782 reverse TSA cases [6, 31] reported a 44 % incidence of postoperative problems and a 24 % complication rate. However, this study included revision procedures, which showed a much higher complication rate than primary procedures [3, 5, 31].
Specific problems with reverse TSA include scapular notching (potentially resulting in accelerated implant wear), instability, and acromial fracture.
Concerns regarding the longevity of the implants have led to recommendations that they are only performed in elderly, low-demand patients [4, 7, 32]. However, Reverse TSA has increasingly been used in younger and more active patients because of the other potential advantages including avoiding problems with rotator cuff insufficiency and the ability to address glenoid retroversion. The revision rate for Reverse TSA is similar to that of anatomic TSA in registry studies [3, 7, 33] and the outcomes of Reverse TSA in patients aged <60 years have been reported to be similar to those of older patients [3, 34].
8.3 Extra-articular Conditions
8.3.1 Heterotopic Ossification
8.3.1.1 Definition
Heterotopic ossification (HO) is the deposition of bone in extraskeletal tissue. The presence of the cellular, protein, and mineral components of bone in HO differentiates it from dystrophic calcification, which is deposition of mineral components only [8, 35].
HO adjacent to a joint usually does not directly involve the nearby articular surfaces. Thus, HO is a cause of joint stiffness in the setting of a normal articular surface. HO is most common around the hip, followed by the elbow. Intervention for HO of the shoulder is seldom required. Pansard et al. [9, 36] reported 539 cases of HO associated with neurological injury that required surgical intervention. Only 3.5 % of the procedures were for shoulder involvement. The authors of that study concluded that HO of the shoulder in this setting was “very rare, or perhaps rarely troublesome” [10, 36].
8.3.1.2 Etiology
HO has been linked to genetic, neurological, and traumatic (postsurgical and postinjury) etiologies. More than one may be present in a single patient.
Congenital
Hereditary HO is a rare genetic disease that has at least three distinct subtypes: Fibro ossificans progressiva (FOP), progressive osseous hyperplasia (POH), and Albright’s Osteodystrophy. Hereditary HO is a progressive disease that usually presents in the first decade of life; however adult-onset FOP has also been reported [8, 37].
Neurological
HO is a relatively common complication following injury to the central nervous system. HO has also been reported following a period of chronic sepsis and coma of 2-weeks duration [11, 12, 38]. The reported incidence is 11–73 % following traumatic brain injury (TBI) and 10–78 % following spinal cord injury (SCI) [9, 39]. The incidence is highest if the joint injury is concomitant to the neurological injury [13, 40].
Traumatic
The incidence of HO following open fractures sustained in combat has been reported to be 38 %, with the hip, elbow, and forearm most commonly requiring treatment [14, 41]. High injury severity scores and injuries to the shoulder, hip, and femur have been shown to be independent risk factors for HO formation in this setting [15, 41].
Postsurgical
The incidence of HO following shoulder arthroplasty has been reported to be between 14 and 54 % [17, 42–45]. Almost all cases were low grade and had minimal functional limitation. Tanner et al. [18, 42] reported that grade III lesions (appearance of complete bone bridge from humerus to glenoid on radiographs) had reduced abduction but no difference in rotation compared to lower grades and shoulders with no HO.
8.3.1.3 Pathology
Heterotopic Ossification
The underlying pathological process that produces HO is poorly understood. Genetic studies of hereditary HO have implicated a receptor for bone morphogenic protein (BMP) type I [19, 37].
Neurological injury has been postulated to activate BMP and prostaglandin (PG) pathways. This induces mesenchymal stem cells in the periphery of the muscle to differentiate into osteoblasts [19, 20, 48]. It has also been that suggested that loss of joint proprioception due to neurological injury has a role in the pathogenesis of HO [21, 49].
Stimulation of mesenchymal stem cells by the local inflammatory response is also thought to be the underlying mechanism of HO due to trauma [22, 39].
Secondary Contracture
Contractures and musculotendinous adhesions can occur in the surrounding soft tissues and can compress surrounding neurovascular structures. As the HO mass slowly grows, a groove forms around these structures and may compress them. Nerve rupture secondary to the HO mass has not been reported.
Risk Factors
There are very little published data regarding the shoulder, but several risk factors for the development of HO about the elbow have been proposed. Patient factors include genetic predisposition, metabolic bone disease, and ankylosing spondylitis. Injury-related factors include severity of initial trauma and concomitant neurological injury. Treatment factors include timing of surgery, surgical approach, and hematoma formation [16, 18, 40]. A delay of ≥8 days from injury to surgery has been reported to have an odds ratio of developing posttraumatic elbow HO that is 12 times the odd ratio for a delay of <1 day. Immobilization >15 days has also been reported to have higher odds of HO compared to immobilization for <7 days [51].
8.3.1.4 Classification
The classification of HO about total hip arthroplasty was described by Brooker in 1973 [52]. This comprised four classes, from islands of bone in the soft tissues to bone spurs that did not connect and finally to apparent anklylosis. Kjaersgaard-Andersen et al. [43] used a similar concept to classify HO about total shoulder arthroplasty (Table 8.1).
Grade | Description |
|---|---|
0 | No ossification |
I | <50 % of the spacea |
II | >50% but <100 % |
III | Ossifications roentgenographically bridging the spacea |
8.3.1.5 Clinical Presentation
History
Examination
The patient may show evidence of prior trauma such as scars (burns, wounds, or surgical) or deformity. The affected joint may be swollen in the case of neurological injury. However, the most common examination finding is a loss of both passive and active movement.
Investigation
Plain radiographs will reveal the HO lesion (Fig. 8.4 ). Proper orthogonal views may not be possible because of inability to position the limb appropriately due to the contracture.
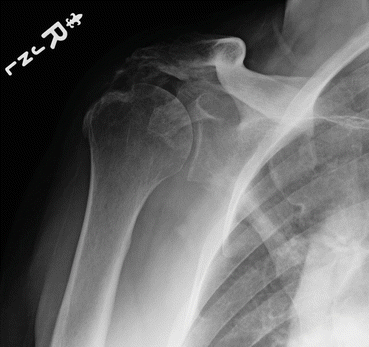

Fig. 8.4
Plain radiograph of the shoulder demonstrating heterotopic ossification between the humeral head and acromion (Image courtesy of Dr Felix Savoie III. Used with permission)
CT scans with three-dimensional (3D) reconstructions are useful for defining the extent and morphology of the HO lesion if surgical treatment is being considered. The proximity of neurovascular structures can also be assessed [36].
Neurological disturbance due to HO is almost always due to compression and stretch caused by the mass effect of the lesion. Nerve rupture due to HO has not been reported. Nerve conduction and electromyography (EMG) studies are only required if there is clinical evidence of a preoperative axillary nerve injury [36].
8.3.1.6 Treatment
There is comparatively little published literature on the management of HO affecting the shoulder. The majority of published treatment recommendations have been extrapolated from data published on the hip and, to a lesser extent, the elbow. In reality, HO of the shoulder is rarely a problem and treatment of any kind is seldom required.
Prophylaxis
Primary Prophylaxis
Primary prophylaxis as treatment may be given to patients with no evidence of HO but a high risk of developing the problem (e.g., open fractures).
Primary prophylaxis regimes have been shown to be effective in the hip [53] but not the upper limb. Radiotherapy has been associated with nonunion following fixation of humerus fractures [54], and NSAIDs have not shown to be of any benefit for preventing HO following primary shoulder arthroplasty [43, 55].
Secondary Prophylaxis
Secondary prophylaxis is treatment given to prevent recurrence of HO following surgical excision [56].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








