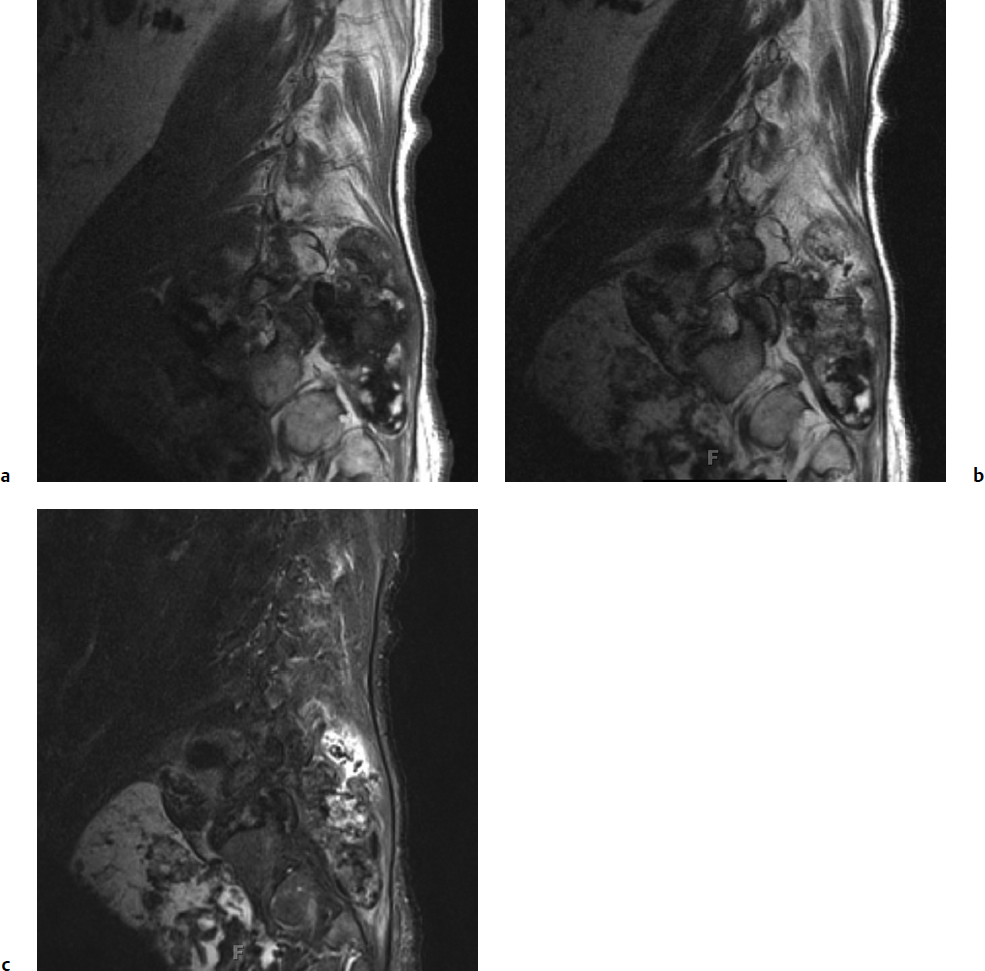
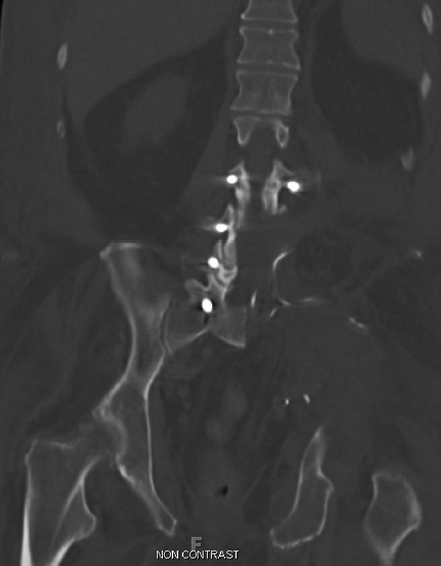
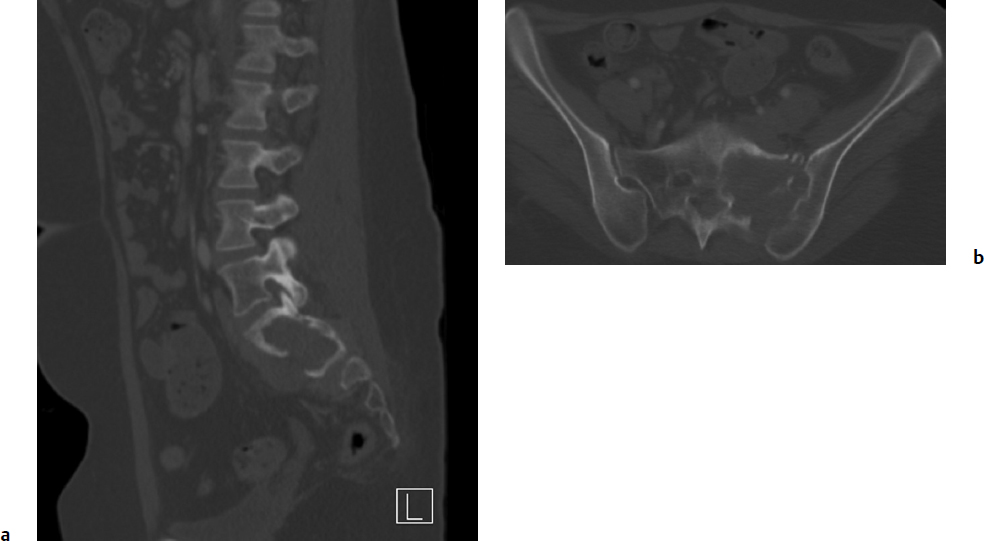
10 Osteogenic sarcoma and Ewing’s sarcoma are malignant tumors that generally arise in the appendicular skeleton, but in rare cases occur as primary tumors of the spinal column. Together with chondrosarcoma, these three sarcomas account for 90% of all primary sarcomas of the bone.1 Classic osteogenic sarcoma and Ewing’s sarcoma are by definition high-grade tumors, and, left untreated, they are associated with aggressive local and metastatic spread. The goals of management are to control localized disease and prevent metastatic spread, which may be accomplished by utilizing a combination of chemotherapy, radiation, and surgery. Surgical control of malignant tumors in the spinal column presents a complex challenge due to anatomic constraints that make it difficult to obtain a wide margin of excision.2 Diagnostic and Management Strategies This chapter reviews the epidemiology, clinical presentation, radiographic findings, pathology, and recommended multidisciplinary management options of the most common sarcomas affecting the bone: osteogenic sarcoma and Ewing’s sarcoma. Chondrosarcoma of the spine is discussed in Chapter 9. Osteogenic sarcoma is a primary malignant tumor of connective tissue origin, characterized by bone or osteoid matrix formation within the tumor mass. There are many tumor variants with different morphologies, with the most common type being classic osteoblastic osteogenic sarcoma. It is the most frequent malignant condition of the bone, accounting for 35% of primary bone malignancies.6 Within the spine, osteogenic sarcoma is the most common sarcoma and makes up 3 to 15% of all primary spinal tumors.7,8 The peak incidence of osteogenic sarcoma in the spine occurs in the fourth decade of life.9,10 In contrast, most patients with osteogenic sarcoma in the long bones are under the age of 30. Primary osteogenic sarcoma rarely arises from the cervical spine and occurs at a proportionally higher frequency in the thoracic spine and sacrum, with the sacrum being the primary site in 68 to 75% of patients.7,10 According to an analysis of 430 patients with spinal osteogenic sarcoma from the Surveillance, Epidemiology, and End Results (SEER) database maintained by the National Cancer Institute, the incidence is essentially equal between the sexes (1.2 to 1), and spinal tumors are rare in people of Asian, Native American, and African descent.10 Other conditions associated with increased risk of developing osteogenic sarcoma include Paget’s disease of bone, enchondromatosis, hereditary multiple exostoses, and fibrous dysplasia.4 Degeneration into a pagetoid osteosarcoma occurs in less than 1% of patients, and of those cases only 6% develop in the vertebrae or sacrum.11 Nonetheless, these cases have been documented to account for as much as half of the spinal osteogenic sarcoma cases in some tumor databases.8 Another important risk factor for osteogenic sarcoma is prior radiation exposure, with those subjected to high doses at a younger age having an elevated risk.12 The most frequent presenting symptom is axial or radicular pain.2,7 Neurologic deficits are the second most frequent presenting symptom and can occur in over 50% of patients.7,13 Bowel and bladder symptoms are less common, and usually present later as the tumor progresses.2 In the Cooperative Osteosarcoma Study Group trial, the median delay in diagnosis from the onset of symptoms was 5 months.7 According to the SEER database, 28% of patients diagnosed with osteogenic sarcoma of the spine presented with gross metastatic disease.10 The role of computed tomography (CT) and magnetic resonance imaging (MRI) has become increasingly important in the diagnosis and staging of spinal osteogenic sarcoma. Conventional plain radiographs are usually obtained prior to oncological referral, and are useful in determining the aggressiveness of a bone lesion. Plain radiographs typically reveal a dense, solid, and smooth appearance of osteogenic sarcoma.9 However, the findings may be variable, ranging from a osteosclerotic appearance in 20% of patients to a seemingly normal radiograph.2,7 For this reason, CT is the recommended modality for diagnostic imaging of osteogenic sarcoma, and scans show significant matrix calcification in 80% of cases.14 On MRI, the solid, nonmineralized portions of the tumor are hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences9 (Fig. 10.1). There may be focal regions of hypointensity on all pulse sequences, and fluid–fluid levels are common in the telangiectatic subtype.15 In contrast, mineralized tumors appear isointense to bone on T1- and T2-weighted sequences4 (Fig. 10.1). Edema in the surrounding tissues is often seen, but is not specific for osteogenic sarcoma.9 Fig. 10.1a–c Sagittal T1 (a), T2 (b), and fat-suppressed (c) magnetic resonance imaging (MRI) scans demonstrating large sarcoma of the left hemipelvis extending into the left retroperitoneum, with extension into the left psoas, iliacus, and iliopsoas muscles. The lesion is heterogeneously enhancing secondary to inconsistent mineralization of the tumor. The majority of osteogenic sarcoma patients who present with metastatic disease have detectable lung metastases, and a CT of the chest, abdomen, and pelvis can assist with tumor staging.16 Chest CT is the most sensitive imaging technique in detecting lung metastases, and should be used when available.16 A technetium-99m methylene diphosphonate (99mTc) bone scan may be necessary to identify additional bone metastases and skip lesions. Grossly, osteogenic sarcoma exhibits a red, gritty, granular quality due to osteoid formation.4 Foci of hemorrhage or necrosis are common histological findings.4 The majority of osteogenic sarcomas are by definition high grade and have a medullary origin.17 On a cellular level, osteogenic sarcoma is characterized by spindle cells with nuclear pleomorphism.18 The histological hallmark of osteogenic sarcoma is the production of bone or osteoid matrix within the tumor, and is the key to diagnosis.9 Conventional osteogenic sarcoma cells produce different types of extracellular matrix, hence the division into osteoblastic, chondroblastic, and fibroblastic subtypes.18 However, most tumors do not sort neatly into a subcategory and present with a mixed histology, and there is no significant difference in prognosis or treatment between the subtypes.17 Other rare but notable histological types include telangiectatic, small-cell, and epithelioid osteogenic sarcomas. Telangiectatic osteogenic sarcoma shares features with aneurysmal bone cysts, and the lesion is composed of multiple blood-filled sinusoids.17 With the application of modern-era chemotherapy agents, the prognosis for telangiectatic osteogenic sarcoma has improved and now carries a prognosis similar to that of the conventional type.18 Small-cell osteogenic sarcoma may be confused with Ewing’s sarcoma due to its round, hyperchromatic nuclei and CD99 positivity.17 Further, translocations mimicking Ewing’s sarcoma between chromosomes 11 and 22 have also been observed17 (see Histopathology, in the Ewing’s Sarcoma section of the chapter, below). Finally, epithelioid osteogenic sarcomas are poorly differentiated and may resemble carcinomas.17 Currently, there are no standard oncological staging scales used for osteogenic sarcoma. Application of bone tumor staging systems, such as those from the Musculoskeletal Tumor Society and the American Joint Committee on Cancer are limited in efficacy due to the high-grade nature of osteogenic sarcoma and its lack of lymph node involvement.1 Further, the unique anatomic constraints for patients with osteogenic sarcoma of the spine compared with that of the extremities may limit the application of a generalized grading scheme. Recently, a grading scale for malignant osseous spinal neoplasms, including osteogenic sarcomas, has been proposed in order to enhance risk stratification of treatment candidates.19 The most important prognostic variables are patient age, metastatic status, and extent of local tumor invasion.19 The gold standard of treatment for patients with localized osteogenic sarcoma involves a multidisciplinary approach, consisting of neoadjuvant chemotherapy with wide local surgical excision. The landmark data for multimodal treatment of osteogenic sarcoma of the limbs was provided by Link et al,20 in which patients undergoing limb resection were randomly assigned to adjuvant chemotherapy or observation only. The relapse-free survival at 2 years was statistically significant between the two cohorts, with 17% of the control group achieving relapse-free survival compared to 66% in the adjuvant chemotherapy group. Gherlinzoni et al21 performed a retrospective review on 355 patients with osteogenic sarcoma of the limbs, and found that the incidence of local recurrence was related to surgical margin and to tumor necrosis induced by neoadjuvant chemotherapy. Chemotherapy has also been shown to provide improved local control and survival for patients with osteogenic sarcoma of the spine, although high-quality studies are lacking.7,13,22 Current neoadjuvant chemotherapy protocols use a combination of methotrexate, cisplatin, doxorubicin, ifosfamide, BCD (bleomycin, cytoxan, dactinomycin), etoposide, and muramyl tripeptide.6 Shives et al13 evaluated 27 patients with spinal osteogenic sarcoma and found a longer survival in patients with adjuvant therapy, although no statistical analysis was performed. Reflecting these results, the 2009 consensus recommendation of the Spine Oncology Study Group (SOSG) stated that neoadjuvant chemotherapy offers significant improvements in local control and long-term survival for spinal osteogenic sarcoma.6 The only effective surgical treatment of localized spinal osteogenic sarcoma is total spondylectomy or wide local excision with vertebral column reconstruction4 (Fig. 10.2), Although the strongest evidence for aggressive surgery derives from treatment of patients with limb tumors, as evidenced by Link et al20 and Gherlinzoni et al,21 there is evidence of spinal osteogenic sarcoma as well. DeLaney et al23 retrospectively reviewed 41 patients with osteogenic sarcoma, including eight patients who had a spinal tumor. The authors found a higher local control rate for patients undergoing gross total resection with negative versus positive margins (78% vs 68%), although the difference was not statistically significant. Fig. 10.2 Coronal computed tomography (CT) demonstrating an L4-5 vertebrectomy and hemipelvectomy with hardware reconstruction. Ozaki and colleagues7 evaluated 22 patients with osteogenic sarcoma of the axial spine. There was a significant survival difference between five patients who underwent wide or marginal surgery and 17 patients who did not, leading the authors to conclude that at least marginal excision is warranted in potentially resectable tumors. Likewise, Sundaresan et al22 reviewed the results of 24 patients with spinal osteosarcoma who underwent aggressive treatment (aggressive resection, radiation, and chemotherapy) compared with patients who underwent less aggressive treatment (limited resection and radiation). The authors found that patients with a more aggressive treatment had an improved survival, although no statistical analysis was done due to the low sample size. More recently, Mukherjee and colleagues24 examined 158 patients in the SEER database with localized spinal osteogenic sarcoma and found a threefold survival (hazard ratio [HR], 0.382; 95% confidence interval [CI], 0.21–0.69) for patients with surgical resection over biopsy alone. The dramatic enhancement in survival was independent of other variables such as tumor location or patient age; however, the extent of resection and surgical margins were not available in the SEER database. The SOSG strongly recommends the use of en-bloc resection of osteogenic sarcoma with wide margins, as it provides improved local control and potentially improved survival.6 The morbidity and mortality of en-bloc procedures are considerable, and they should be performed only at experienced centers with multidisciplinary teams. The overall 5-year survival rate in all patients who have spinal osteogenic sarcoma is 18%.10 The estimated median survival in patients who present with localized disease and receive aggressive treatment is 18 months.10 An estimated 7% of patients with spinal osteogenic sarcomas present with metastatic disease, and have an approximate median survival of 7 months.10 Radiation therapy may play a role in palliative care for those with unresectable or metastatic disease; however, osteogenic sarcomas are radioresistant and do not respond well to standard doses.4 An improvement in survival rates has also been associated with the use of radiation when combined with surgery.23,24 This may be due to the efficacy of radiation treatment in treating microscopic or minimal residual disease after surgery. Ewing’s sarcoma represents a family of neoplasms originating from a precursor neural cell, including classic Ewing’s sarcoma and primitive neuroectodermal tumor.4,25 Ewing’s sarcoma is the second most common cancer of bone in adolescents and young adults, after osteogenic sarcoma, with an incidence of 2.1 per 1 million children in the United States.26 This sarcoma of the spine tends to present at a young age, with a peak incidence in the second decade and an age range spanning the first through third decades.9 There is a slight male predominance in the spine (1.8 to 1). The condition is exceedingly rare in people of Asian, Native American, or African ancestry.10 The cause of Ewing’s sarcoma is not clear, and is most likely due to spontaneous genetic translocations rather than familial or environmental factors.25 Further, unlike osteosarcoma, there is no associated risk from prior exposure to radiation.4 The spine is an uncommon site, with only 3 to 15% of Ewing’s sarcoma occurring in the spine.2 However, Ewing’s sarcoma is the most common malignant vertebral tumor found in children.27 It can affect any segment of the spine, as well as originate within the paravertebral muscles and invade into the epidural space.4 Within the axial skeleton, Ewing’s sarcoma is most commonly found in the sacrum and lumbar spine, with common involvement of the sacral ala and posterior elements, respectively.3 Within the sacrum, the tumor may grow to a large size before the onset of pain. Therefore, the insidious malignancy may remain undetected for a prolonged period of time.4 On presentation, the primary symptom of spinal Ewing’s sarcoma is localized pain with variable intensity, which occurs in virtually all patients.2 This pain is often mistaken for normal growing pains or sports-related injuries in the adolescent population.25 Neurologic deficits are often seen, occurring in 40 to 60% of patients.2 Bowel and bladder symptoms are rare and tend to occur later in the disease progression.2 Systemic symptoms such as fever and indicators of a chronic inflammatory state may also occur and lead to a mistaken diagnosis of infection.4,25 These fairly nonspecific symptoms commonly lead to a delay in the establishment of diagnosis, allowing the malignant tumor to progress. According to the SEER database, 34% of 430 patients with Ewing’s sarcoma of the spine present with gross metastatic disease.10 The most important diagnostic imaging modalities for evaluation of potential Ewing’s sarcoma are CT and MRI. Unlike osteogenic sarcoma, plain radiographs are rarely useful in diagnosing Ewing’s sarcoma, as this disease process causes a “moth-eaten” destruction of bone rather than complete osteolysis.2,15 Radiographic images of early disease can range from a seemingly negative radiograph to subtle findings such as osteolysis and haziness of the end plates.28 Computed tomography is a useful complementary modality to evaluate the bony elements affected by Ewing’s sarcoma. An osteolytic mass is detected in approximately 90% of cases, with rare variants that may present with a mixed lytic/sclerotic or purely sclerotic morphology.28 The majority of tumors in the mobile spine involve the posterior elements, whereas most tumors in the sacrum involve the sacral ala28 (Fig. 10.3). A CT of the chest, abdomen, and pelvis is recommended for surgical staging. The most common sites for metastatic disease are the lungs, bone, and bone marrow. Further, a 99mTc bone scan demonstrates intense tracer uptake in Ewing’s sarcoma.9,16 The recommended modality to evaluate Ewing’s sarcoma is MRI with gadolinium enhancement, which enables sufficient evaluation of the soft tissue mass and its surrounding anatomy (Fig. 10.4). The tumor bulk is isointense to bone marrow on T1-weighted sequences and isointense to hyperintense to bone marrow on T2-weighted sequences.9 The cortex of the bone is usually preserved, and patients may present with a pathological fracture if the lesion occurs within the vertebral column.15 Grossly, Ewing’s sarcoma appears as a gray-white tumor with areas of hemorrhage and necrosis.3 Evidence of necrosis and atypical histological features have been found to have worse prognostic value in patients with primary Ewing’s sarcoma of the extremities.4 On a microscopic level, Ewing’s sarcoma consists of small, round cells with oval, uniform nuclei.9,18 The cellular boundaries are indistinct, and give the appearance of a syncytium with multiple nuclei.9 Occasionally present are mitotic figures, with apoptotic and karyopyknotic cells.3 The differential diagnosis for small, round cell tumors includes lymphoma of bone, metastatic neuroblastoma, embryonal rhabdomyosarcoma, small cell osteosarcoma, and osteomyelitis; thus, diagnosis of Ewing’s sarcoma is difficult on light microscopy alone.1,4
Osteogenic Sarcoma and Ewing’s Sarcoma of the Spine

 Introduction
Introduction
 Definitive diagnosis is needed in order to start patients on neoadjuvant chemotherapy prior to definitive local control for Ewing’s sarcoma and osteosarcoma.
Definitive diagnosis is needed in order to start patients on neoadjuvant chemotherapy prior to definitive local control for Ewing’s sarcoma and osteosarcoma.
 Fine- or core-needle biopsy are appropriate methods if a specific diagnosis is suspected and a limited amount of tissue is required for diagnosis.3 If an open biopsy is required, the incision should be permanently marked to facilitate a wide excision at another time if necessary.3
Fine- or core-needle biopsy are appropriate methods if a specific diagnosis is suspected and a limited amount of tissue is required for diagnosis.3 If an open biopsy is required, the incision should be permanently marked to facilitate a wide excision at another time if necessary.3
 When metastatic disease is present, consultation with a medical oncologist and radiation oncologist is helpful to determine if the patient is a surgical candidate. If there is direct soft tissue extension with bowel involvement, consultation with a general surgeon may further facilitate surgical planning.
When metastatic disease is present, consultation with a medical oncologist and radiation oncologist is helpful to determine if the patient is a surgical candidate. If there is direct soft tissue extension with bowel involvement, consultation with a general surgeon may further facilitate surgical planning.
 En-bloc procedures are designed to achieve wide margins, and the three main techniques in the spine are spondylectomy, sagittal resection, and resection of the posterior arch.
En-bloc procedures are designed to achieve wide margins, and the three main techniques in the spine are spondylectomy, sagittal resection, and resection of the posterior arch.
 Sagittal resections involve a wedge resection of the vertebral body with excision of the posterior elements, and are ideal in situations when tumor is confined to an eccentric portion of the vertebral body, pedicle, or transverse process.3
Sagittal resections involve a wedge resection of the vertebral body with excision of the posterior elements, and are ideal in situations when tumor is confined to an eccentric portion of the vertebral body, pedicle, or transverse process.3
 En-bloc resection of the posterior arch is performed only when the tumor is located in the posterior arch without pedicle involvement.
En-bloc resection of the posterior arch is performed only when the tumor is located in the posterior arch without pedicle involvement.
 Spondylectomy involves the removal of the entire tumor in one piece together with portions of the posterior elements.4 This is the procedure of choice when the tumor is centrally located and involves no more than one pedicle.3
Spondylectomy involves the removal of the entire tumor in one piece together with portions of the posterior elements.4 This is the procedure of choice when the tumor is centrally located and involves no more than one pedicle.3
 Previous data from our group has demonstrated success with posterior-only approaches for en-bloc resection of sacral tumors.5
Previous data from our group has demonstrated success with posterior-only approaches for en-bloc resection of sacral tumors.5
 If more than half of the sacroiliac joint is removed for tumor resection, hardware reconstruction with a transiliac bar or femoral allograft is required.
If more than half of the sacroiliac joint is removed for tumor resection, hardware reconstruction with a transiliac bar or femoral allograft is required.
 Lesions not involving the sacroiliac joint can be removed with a low sacral amputation without any hardware reconstruction.
Lesions not involving the sacroiliac joint can be removed with a low sacral amputation without any hardware reconstruction.
 Osteogenic Sarcoma (Osteosarcoma)
Osteogenic Sarcoma (Osteosarcoma)
Epidemiology
Clinical Manifestation
Radiographic Features
Histopathology
Management
 Ewing’s Sarcoma
Ewing’s Sarcoma
Epidemiology
Clinical Manifestation
Radiographic Features
Histopathology
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree