Osteochondroses of the Foot and Ankle
Lawrence M. Fallat
Jeffrey C. Christensen
Jacob A. Hord
The term osteochondrosis has been used for a variety of painful disorders involving an actively growing epiphysis or an apophysis. At one time, all of these disorders were thought to be related to trauma with a subsequent avascular necrosis. Today, disorders that are believed to involve an osteonecrosis include Freiberg disease, Mueller-Weiss syndrome, Buschke disease, osteochondral lesions of the talus, and possibly Köhler disease. Sever and Iselin diseases result from repetitive stress to the secondary growth center or, in the case of Iselin disease, a normal variant of enchondral bone formation.
OSTEOCHONDRAL LESIONS OF THE TALUS
Transchondral lesions represent a localized injury to the articular surface of a joint as well as a corresponding portion of subchondral bone. In the ankle joint, the injury is usually represented by the compression or separation of a fragment from the dome of the talus. If healing does not occur following the injury, then the fragment may undergo avascular necrosis. These lesions can be a source of significant pain and disability. Once thought to be uncommon, transchondral lesions of the talus are being diagnosed with greater frequency as a result of increased physician awareness and more accurate diagnostic imaging techniques.
Early investigators who had observed loose bodies in joints speculated about the possible factors that would cause a small fragment of bone and cartilage to separate from the articular surface. The first case of a loose bone fragment in the ankle joint was reported by Alexander Munro in 1856. Although no attempt was made to classify the process, he did believe that the lesion was the result of an injury (1). Konig, in 1888, appears to have started the controversy regarding the etiology of these lesions when he reported on several unattached bodies in the knee joint (2). In that era, these fragments were referred to as arthrophytes or corpora mobile (3). Konig believed that these lesions could not have been caused by any known disease, tumor, or traumatic event and decided that the etiology was due to an inflammatory process of the joint surfaces that resulted in a spontaneous necrosis of a portion of bone. He termed these lesions “osteochondritis dissecans” (2). The word “osteochondritis” referred to the inflammatory process in the joint and “dissecans” is derived from the Latin word dessec, which means “to separate” (3). Later investigators would find no inflammatory cells in the fragments; however, the term of osteochondritis has persisted to the present day (3).
Kappis (4), in 1922, was the first to apply the term osteochondritis dissecans to the ankle joint. Rendu (5), in 1932 reported a case of a loose body in the ankle joint that he called an intraarticular fragmentary fracture of the talus, indicating his belief that trauma was the origin of the lesion. The lesion Rendu described was based on radiographs and appears to be the same as those described by Munro (1) and Kappis (4). Fairbanks (6), in 1933, wrote that trauma without any underlying vascular disturbances was the only etiology of loose intraarticular bodies. In 1953, Roden et al (7) presented the first thorough study by reviewing 55 cases of osteochondral lesions of the talus. A considerable amount of information was obtained from their investigation that would prove valuable in understanding this condition. They determined that the lateral lesions were more often due to trauma, rarely healed spontaneously, and created more symptoms than those found medially. Surgical treatment was recommended for the lateral lesions because they frequently resulted in arthritic changes in the joint (7).
A greater understanding of the osteochondral lesions was obtained in 1959 with the study by Berndt and Harty (8). The investigators determined experimentally, using cadaver limbs, that trauma resulted in localized fractures of the talar dome. They were also able to define the mechanism of injury of both medial and lateral lesions. Attempting to reflect the etiology of trauma and the location of the injury on the talar dome, they introduced the term “transchondral fracture” to describe osteochondral lesions in this site. O’Donoghue (9,10 and 11) published several articles discussing osteochondral fragments in the knee and supported the concept of a traumatic etiology. Much of what he had written was believed to apply to the lesions found in the ankle as well (12).
Despite the fact that antecedent trauma appears to be the etiology for most lesions, others continued to believe that there could possibly be an alternative etiology because of the many reports of transchondral lesions without a history of injury (13). Some authors believed that lateral lesions were likely to be created by traumatic events but questioned the etiology of medial talar dome lesions (7,14). Flick and Gould (15) reviewed the medical literature and determined that 98% of lateral lesions and only 70% of the medial lesions were reported to have been caused by trauma. Campbell and Ranawat believed that ischemic changes may play a role in the development of osteochondral lesions. They were able to demonstrate, in their specimens, that bone infarction preceded pathologic fracture through the subchondral bone and suggested that repeated minor episodes of trauma may result in fracture and eventual separation of the osteochondral fragment (16).
Others have proposed that there are two forms of osteochondritis. One type develops in older children and adults and is attributable to a traumatic event. Another form, occurring in children, is due to localized avascular necrosis. Smith (17) noted that these lesions were often multiple and sometimes familial. Smillie also believed that there was both an adult and juvenile form of osteochondritis. It was proposed that in the juvenile form, there was an underlying abnormality of ossification, most often of enchondral dysostosis, leading to the development of an accessory ossification center. If subsequent trauma disturbed the blood supply to this area, the resultant ischemia resulted in osteochondritis (18).
Other potential etiologies for osteochondritis that have been proposed have included a congenital predisposition to
aseptic separation of the articular osteochondral fragment (19), other congenital or hereditary factors (20), embolism of the epiphyseal arteries (21), endocrine imbalance at the time of puberty (22), and developmental abnormalities (23). Axhausen (24) believed that impaction from opposing articular surfaces damaged blood vessels leading to necrosis of the area supplied by these vessels. This led to absorption, separation, and eventual extrusion of the dead portion of bone into the joint. In one study evaluating the family members of 34 patients with osteochondritis dissecans and 86 of their firstdegree relatives, only 1 additional individual was found to have osteochondritis dissecans. Association with other forms of osteochondritis dissecans, endocrine disorders, and dwarfism could not be demonstrated (25).
aseptic separation of the articular osteochondral fragment (19), other congenital or hereditary factors (20), embolism of the epiphyseal arteries (21), endocrine imbalance at the time of puberty (22), and developmental abnormalities (23). Axhausen (24) believed that impaction from opposing articular surfaces damaged blood vessels leading to necrosis of the area supplied by these vessels. This led to absorption, separation, and eventual extrusion of the dead portion of bone into the joint. In one study evaluating the family members of 34 patients with osteochondritis dissecans and 86 of their firstdegree relatives, only 1 additional individual was found to have osteochondritis dissecans. Association with other forms of osteochondritis dissecans, endocrine disorders, and dwarfism could not be demonstrated (25).
INCIDENCE
Transchondral lesions have been reported to compose 0.09% of all fractures (26) and 1% of all talar fractures (27). The prevalence has been estimated to be 0.002 cases per 1,000 people (28). Osteochondritis dissecans of the talus accounts for 4% of all reported cases of osteochondritis (29) and osteochondral lesions of the talus are the second most common location for this process after the lateral aspect of the medial epicondyle of the knee (30).
The number of reported cases of transchondral lesions is probably well below the actual incidence. In one study, emergency room physicians misdiagnosed seven of 16 patients as having a sprained ankle, yet retrospective review of the radiographs demonstrated the transchondral fracture (15). In some instances, the average length of time from the onset of symptoms to the diagnosis has been as long as 36 months (31). Transchondral lesions have been found in 6.5% of 133 patients treated for sprained ankles. Most of these injuries occurred while the patients were participating in sports-related activities (32). Therefore, it would seem logical that in some instances, the associated osteochondral fracture would remain undiagnosed without a high index of suspicion.
Transchondral lesions have been reported in both sexes, but a slight majority is seen in males in their second through fourth decades. The average age is about 25 years, although cases have also been reported in patients in their fifth and sixth decades of life (15,29,33,34,35,36,37,38,39,40,41,42 and 43). Although they are not as common, children may also sustain transchondral injuries. Wester et al (44) reported that of 18 children in his study, the ages ranged from 2 to 14 years, with the average age at the time of injury being 9 years. In a similar report involving 18 adolescents with transchondral lesions, the ages of the patients ranged from 9.9 to 16 years, with the mean age being 14.2 years (45). Some controversy exists regarding healing of transchondral lesions in children. It has been proposed that the lesions healed spontaneously (28), but in one report, there were three cases of transchondral lesions in children that did not heal with conservative treatment (15).
CLINICAL PRESENTATION
Because of the chronic nature of many transchondral lesions of the talus and the variation in the severity of damage to the talar dome, there are no specific pathognomonic clinical signs of this condition. Because the mechanism of injury associated with the lesions is inversion of the ankle, transchondral fractures may be suspected in patients with a history of a sprained ankle. A large number of patients who present with talar dome lesions recall a previous ankle injury (31,37), and in some instances, this consisted of an ankle fracture (37).
With recent acute injuries, the patient may present with the usual signs and symptoms of a sprained ankle consisting of pain, swelling, ecchymosis, erythema, and reduced motion as a result of guarding. Gradually, the symptoms associated with the acute ankle sprain will improve, but if a transchondral lesion is present, the ankle may never become completely asymptomatic or the patient may do well for a period of time only to develop later symptoms (45,46).
The symptoms of a chronic transchondral lesion may include pain, swelling, locking of the joint, stiffness, ankle instability (45,46), chronic sprains without locking of the ankle, functional instability of the ankle, and a decreased range of motion with synovial effusion (45). In one large study, the most common symptoms appeared to be pain with activity, daily pain, pain occurring weekly, night pain, and swelling. The physical examination did not prove to be very helpful in diagnosing transchondral lesions. Fifty percent of the patients had a slight decrease in ankle joint range of motion, and plantarflexion was usually reduced. Twenty percent of the patients also had some loss of subtalar joint motion (31).
CLASSIFICATION
The most commonly accepted classification of transchondral lesions is the system developed by Berndt and Harty (Fig. 54.1). Transchondral lesions were divided into four stages: stage I represents a small area of subchondral bone compression, stage II is a partially detached osteochondral fragment, stage III is a completely detached fragment without displacement and remaining in the crater, and stage IV is an osteochondral fragment that is completely detached and displaced from the crater (8). It is difficult to accurately diagnose grade I lesions and to differentiate grades I, II, and III on standard radiographs. In
addition, Loomer et al employing bone scans and computed tomography (CT), noted that in 77% of the patients, the Berndt and Harty system proved inadequate to describe the lesions. Based on their findings, they added a fifth category to the classification that they referred to as a radiolucent defect. This was the most common lesion seen in their study (31).
addition, Loomer et al employing bone scans and computed tomography (CT), noted that in 77% of the patients, the Berndt and Harty system proved inadequate to describe the lesions. Based on their findings, they added a fifth category to the classification that they referred to as a radiolucent defect. This was the most common lesion seen in their study (31).
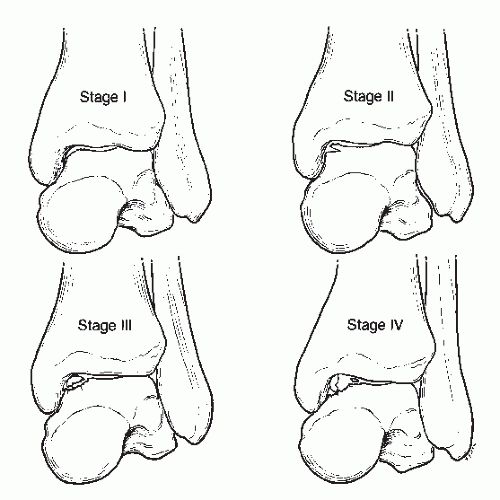 Figure 54.1 The four stages of osteochondral lesions of the talus according to the Berndt and Harty classification. |
Additional classification systems have been developed based on findings noted with magnetic resonance imaging (MRI) and ankle arthroscopy (37,47,48). Pritsch et al developed an arthroscopic grading system that they believed was more significant than the radiographic appearance of the lesions. This system was based on the condition of the articular cartilage of the talus. Grade I is intact, firm, shiny cartilage; grade II is intact but soft cartilage; and grade III is frayed cartilage. The authors determined that an increase in the radiographic stage of the lesion did not necessarily predict increasing fragmentation or loosening of the lesion (48). Ferkel et al (49) developed a CT staging classification: stage I, cystic lesion within the dome of the talus; stage IIA, cystic lesion with communication to talar dome surface; stage IIB, open articular surface lesion with overlying nondisplaced fragment; stage III, nondisplaced lesion with lucency; and stage IV displaced fragment.
Ly and Fallat (37) developed a simplified grading system with a good practical application. A stage I lesion represents a recent fracture of the dome of the talus. The fragment may remain in satisfactory position or may be displaced. Stage II represents an older chronic lesion that has not healed. Avascular necrosis of the subchondral bone has occurred, and this lesion is seen as a lucency on standard radiographs.
LOCATION OF THE LESIONS
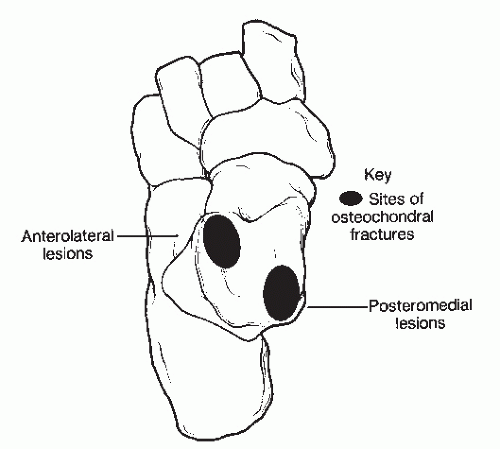
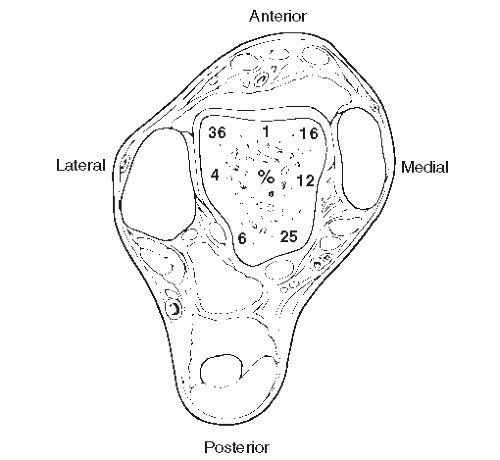
Berndt and Harty were the first to determine the characteristic locations of transchondral lesions of the talar dome. In their cadaveric study, they demonstrated that 43.7% of the lesions occurred laterally, most of which were in the middle third of the talar dome. There was a 56.3% incidence of medial lesions, which were usually located in the posterior third of the medial border of the talar dome (Fig. 54.2) (8). Most investigators have reported a similar incidence and pattern of location, although some authors have found the lateral location to be the most common, and lesions have been noted to develop at different positions on each border of the talar dome (12,14,15,37,50,51). Central transchondral lesions have also been reported, although at a very low incidence (14,52,53), and cases of both medial and lateral lesions in the same ankle have been reported (34,37). Loomer et al (31) found the location of transchondral lesions to be more diverse than had been previously reported, with the location varying around the periphery of the talar dome. The most common sites were the anterolateral quadrant, followed by the posterior medial quadrant (Fig. 54.3) (46). Raikin et al retrospectively reviewed MRI examinations of 424 patients and found that the medial central talar dome was most frequently involved with 53.0% of the lesions located there. The lateral central portion of the talar dome was second representing 25.7% of the lesions (54) (Fig. 54.4).
The frequency of occurrence for each stage of transchondral lesions was usually not reported until recently. Loomer et al (31) reported 2% of the cases in their series were compression injuries, 1% were partial fractures, 13% were complete but undisplaced, and 7% were displaced fractures. Ly and Fallat (37) reported that stage II lesions were the most common of the lateral lesions and stage III were the most frequent medial lesions.
MECHANISM OF INJURY
Lateral transchondral lesions occur when an inversion force is applied to the dorsiflexed foot. The wider anterior portion of the talus becomes wedged in the ankle mortise, resulting in the anterior and middle aspect of the lateral border of the talar dome impacting against the lateral malleolus. This results in a localized area of subchondral bone compression that is characteristic of the stage I lesion. The collateral ligaments need not be ruptured for this lesion to develop. When a greater force is applied, the ligaments rupture, resulting in continued talar inversion and rotation, creating a shearing effect as the talus
abrades the fibula. This may create the characteristic shallow, wafer-shaped lesion that is frequently seen on the lateral aspect of the talus (8). The fragment is lifted up from the lateral surface of the talus, and the fracture extends a short distance across the dome until the force is dissipated or until the fragment is dislodged. There appears to be a greater incidence of stage IV lesions occurring laterally, and the loose fragments may account for the increased symptoms associated with the lateral lesions.
abrades the fibula. This may create the characteristic shallow, wafer-shaped lesion that is frequently seen on the lateral aspect of the talus (8). The fragment is lifted up from the lateral surface of the talus, and the fracture extends a short distance across the dome until the force is dissipated or until the fragment is dislodged. There appears to be a greater incidence of stage IV lesions occurring laterally, and the loose fragments may account for the increased symptoms associated with the lateral lesions.
Medial transchondral lesions involving the posterior third of the talar dome may be produced by inversion and plantarflexory ankle forces, with concomitant lateral rotation of the tibia on the talus. With the foot in this position, talar inversion and rotation result in the medial border of the talar dome impacting against either the medial malleolus or the posterior lip of the tibia. As the force continues, the lateral ankle ligaments become tight and transverse plane motion is converted to sagittal plane motion, resulting in greater impaction of the tibia into the talus (8). Medial dome lesions are typically deep and cup shaped in appearance (Fig. 54.5). As with lateral lesions, a stage I defect can occur without rupture of the collateral ankle ligaments. With further force, the posterior fibers of the deltoid ligament may tear, creating greater instability. Also, the compression of the talus against the tibia may shear an osteochondral fragment from the talus, resulting in the other stages of transchondral lesions (8,34,46).
Centrally located transchondral lesions of the talus are rare, and the mechanism of injury is not fully understood. Theoretically, the central lesion occurs when a load is applied with anterior displacement of the talus or posterior displacement of the tibia, followed by dorsiflexion of the foot (52,53). Sudden impact with the talus in a plantarflexed position and with anterior displacement may result in a central transchondral defect (53).
COURSE OF THE LESION
Inversion ankle sprains appear to be the initiating event that results in osteochondral fractures of the talar dome. In this type of injury, the fracture extends through the cartilage into the subchondral bone, resulting in disruption of the fracture fragment. If the fragment is stable and motion is eliminated, healing may occur when capillary buds cross the fracture line and penetrate into the bone fragment. If there is motion as healing occurs, then fibrous tissue forms at the fracture line that acts as a barrier to prevent capillary ingrowth (8). Thus, the fragment becomes sequestered, essentially resulting in a nonunion. Avascular necrosis soon develops and may be seen on radiographs as a localized area of lucency in the talar dome (Fig. 54.6).
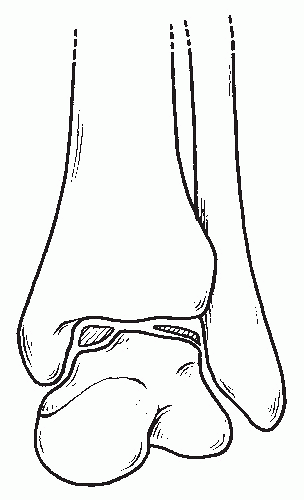 Figure 54.5 The morphologic appearance of lateral versus medial osteochondral lesions. Medial lesions are deep and cup shaped, whereas lateral lesions are shallow and wafer shaped. |
The articular cartilage overlying the talus responds differently to the injury and healing process. Because of the resilience of the cartilage, it may remain attached to the adjacent cartilage,
as seen more commonly in compression injuries, or it may be severely damaged and displaced as in shear-type injuries. In either event, the cartilage overlying the bone fragment may be viable long after the osseous segment has undergone avascular necrosis. The inflammatory phase of healing does not occur in the cartilage, owing to the avascularity of this tissue. However, chondrocytes are able to obtain nourishment from synovial fluid and may remain viable for a considerable period of time (55).
as seen more commonly in compression injuries, or it may be severely damaged and displaced as in shear-type injuries. In either event, the cartilage overlying the bone fragment may be viable long after the osseous segment has undergone avascular necrosis. The inflammatory phase of healing does not occur in the cartilage, owing to the avascularity of this tissue. However, chondrocytes are able to obtain nourishment from synovial fluid and may remain viable for a considerable period of time (55).
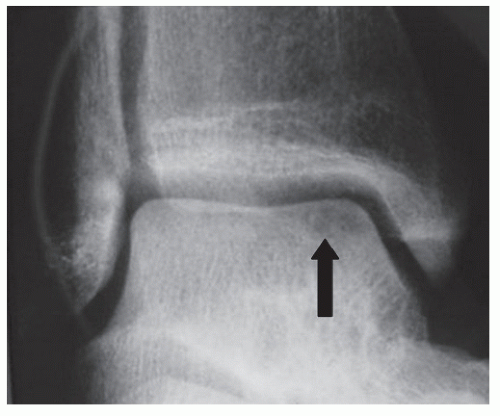 Figure 54.6 Standard radiographs revealing avascular necrosis (arrow) of a medial osteochondral defect. |
Damage to the cartilage at the time of injury, or repeated weight-bearing as subchondral bone becomes necrotic and resorbs, results in uneven loading of the talus, creating even more trauma (56). This problem can result in the cartilage becoming soft, friable, fissured, and discolored, followed by degenerative arthritic changes (12,57).
IMAGING
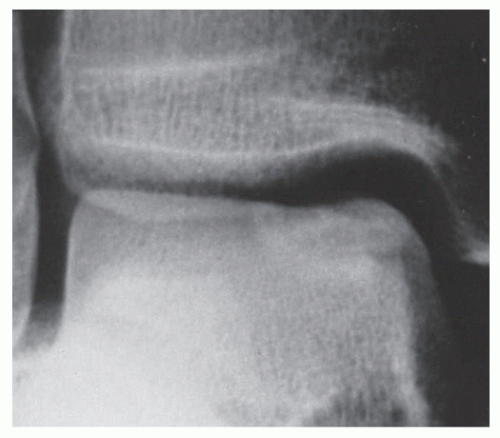
In some instances, radiographs may reveal a transchondral fracture in patients sustaining acute trauma or with more chronic ankle symptoms (31,34,41,46,51,58). However, small subtle lesions may not be visualized on radiographs or may be overlooked. Stage I lesions may not demonstrate any specific changes on standard radiographs (59). The only aberration may be a change in bone density at the margin of the talar dome (60). In many patients, the lesions are not evident on standard radiographs until they have progressed to a more advanced stage (58). Furthermore, standard radiographs may be of little value not only in detecting lesions but also in delineating their size and shape (61) (Fig. 54.7)
Anteroposterior, lateral, and mortise views are recommended for a thorough evaluation of the talar dome. The transchondral lesion is more readily visualized on the mortise view and occasionally on the anteroposterior view (60). The lateral view is rarely useful in diagnosing a transchondral lesion but may reveal synovial effusion, which may be suggestive of this type of process (60). Better visualization of the medial talar dome lesions can be obtained by taking the anteroposterior view with the foot plantarflexed. Lateral lesions can be visualized easier with the foot in the dorsiflexed position (62). Stress inversion views may also aid in revealing talar dome lesions (14,63,64 and 65). In one study, when the talar tilt exceeded 18 degrees, transchondral lesions were noted in 77% of the cases.
When osteonecrosis has developed in stage I, II, or III lesions, radiographs may demonstrate a lucency that can be seen at the apex of the talar dome, at times with a sclerotic layer owing to subchondral bone remodeling (65). Occasionally, there is also a small sclerotic area in the center of the crater that has been referred to as a nidus or the bulls eye deformity. This represents a sequestered portion of the osteochondral fragment that has not yet resorbed (65) (Fig. 54.8).
High-resolution CT has proven to be very reliable in identifying and evaluating transchondral lesions (Fig. 54.9). The quality of the image is superior to tomography (61) and CT also provides a better contrast between bone and soft tissue (61,66). The image cuts are made at 2-mm intervals so that one is provided an image that reflects the true dimensions of the lesion (40). This allows the physician to determine the exact size, shape, and location of the lesion. Plain radiographs project a smaller size of the defect (43). CT can also be obtained while the patient is wearing a cast (37).
MRI also provides cross-sectional images of the talus and offers a precise, noninvasive, nonradiating evaluation of the location and size of the transchondral lesion (Fig. 54.10) (61,67). A unique feature of MRI is that it provides information relative to the stability of the lesion and permits evaluation of the articular cartilage (15,48). Partially attached grade II transchondral lesions demonstrate an irregular high signal zone on the T2-weighted images at the fragment and talar body junction. The completely separated fragment associated with stage III injuries demonstrates a ring of fluid surrounding the lesion (35). Ankle arthroscopy and arthrotomy have confirmed the accuracy and efficacy of MRI in differentiating stage II from stage III lesions (47,66,67).
Although MRI has been used to determine the stage of an osteochondral lesion and evaluate the articular cartilage, it does not define the cortex as well as CT. There have been many comparisons in resent literature of CT, MRI and diagnostic arthroscopy, with CT and MRI, yielding similar
results (59,68,69). MRI has been shown to have a slight diagnostic advantage when radiographs and clinical findings are not diagnostic, and CT is preferred if an osteochondral lesion has been diagnosed (58,68).
results (59,68,69). MRI has been shown to have a slight diagnostic advantage when radiographs and clinical findings are not diagnostic, and CT is preferred if an osteochondral lesion has been diagnosed (58,68).
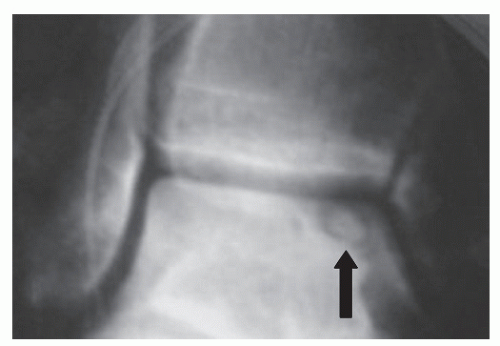 Figure 54.8 Typical nidus (arrow). This represents a portion of cortical bone that has not completely resorbed. |
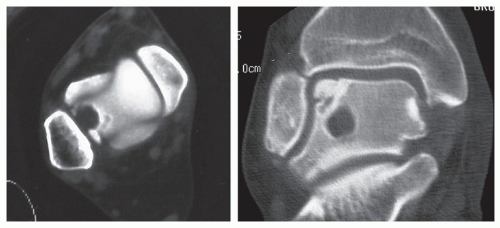 Figure 54.9 CT images are an excellent means of assessing talar dome lesions; they clearly define the location and size of the lesion. |
Triphasic bone scans may also be an effective technique when radiographs are negative but there is still a suspicion of a transchondral lesion (47,64). Bone scans are highly sensitive, yet they are not specific for the type of osseous pathology that may be present (Fig. 54.11). Neoplasms, avascular necrosis of the talus, and infection result in increased uptake of the radiopharmaceutical (60). The presence of hyperemia in the blood pool phase has been believed to be 100% sensitive and 83% specific for osteochondral fractures of the talus (70). Bone scans are very accurate if they are performed at least 48 hours after the injury, but when they are performed within 48 hours following injury, they can yield false-negative results (61). A positive bone scan may confirm the presence of osseous pathology to the talus, and additional testing with CT or MRI may be necessary to further evaluate the talar dome lesion (64).
TREATMENT
In general, most authors treat stage I and stage II lesions conservatively regardless of their location. This is generally true for stage III medial lesions as well. Conservative treatment consisting of reduced activity and limited ankle motion has been advocated for acute stage I lesions (56). Casting would also be appropriate for this stage and has generally been recommended for acute stage II lesions (14,58). However, treatment criteria for stage III transchondral lesions appears to be more controversial, with recommendations ranging from long periods of conservative care to immediate surgery. Canale and Belding (14) recommended surgery for all stage III lateral transchondral lesions but believed that stage III medial lesions should be treated conservatively with casts, patellar braces, ankle corsets, or arch supports. However, if symptoms persisted, surgery should be performed consisting of excision and curettage of the lesion. Loomer et al (31) recommended surgical intervention for chronic stage III lesions consisting of excision if the fragment was small and fixation for larger fragments. Flick and Gould (15) reported that patients treated conservatively for stage III lesions had reduced levels of athletic activity, whereas those who had surgery returned to their preinjury level of activity. They recommended that all symptomatic stage II and stage III lesions that have not responded to conservative care should be treated surgically. However, it would appear appropriate to consider early operative intervention in lateral stage III lesions. All investigators seem to agree that both medial and lateral stage IV lesions should be treated surgically. This typically consists of removal of loose bodies, and in chronic lesions, drilling and curettage of the defect or reduction with fixation of large fragments seen in acute injuries (15,41,48,51,58,63,71). Chen and
Wertheimer (53) recommended early surgical intervention for central transchondral lesions because of the high level of compression transmitted through the central portion of the talar dome.
Wertheimer (53) recommended early surgical intervention for central transchondral lesions because of the high level of compression transmitted through the central portion of the talar dome.
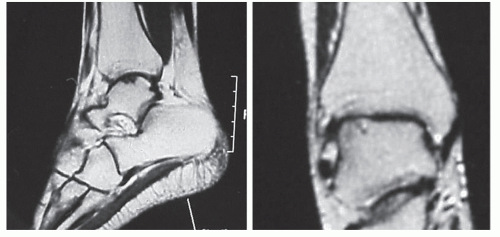 Figure 54.10 MRIs of an osteochondral lesion of the talus. This imaging modality is valuable in providing the specific location and size of the lesion and integrity of the articular cartilage. |
Surgical Procedures
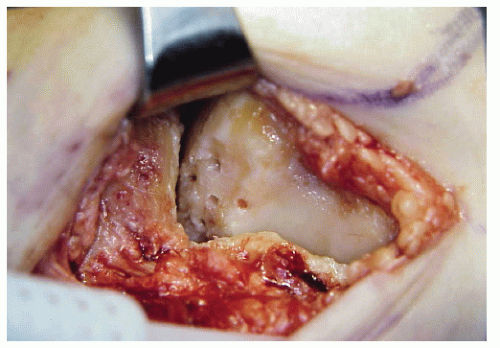
Although several different procedures have been described for the treatment of transchondral lesions of the talus, the approach has typically consisted of curettage and drilling of the defect with removal of any displaced bone fragments from the joint (Fig. 54.12). The cartilage is sharply demarcated with a scalpel applied perpendicular to the articular surface. Drilling of the subchondral bone is performed to encourage vascular ingrowth. Berndt and Harty (8) noted that the osteochondral defect does fill with blood, resulting in the formation of connective tissue that is firm and smooth. Others have confirmed the presence of a firm fibrocartilage tissue in follow-up arthroscopic evaluation of the lesions (36,51). Because of good results reported with this procedure, curettage and drilling has become an accepted treatment for transchondral lesions. Overall, the results of surgery for transchondral lesions have generally been reported to be good (8,12,14,15,31,33,36,51,63).
Some variations of this procedure have been reported. Other surgeons have proposed that just drilling into the subchondral bone would be sufficient if the articular cartilage was still intact (33). Loomer et al (31) recommended drilling a small hole in the intact articular cartilage to aid in locating the radiolucent type of lesions. Once located, the fibrous tissue is removed through the drill hole, then three or four small 2-mm diameter holes are drilled through the cartilage into the trabecular bone. Good results have also been reported with sharp excision of the osteochondritic lesions and drilling of the lesions (68).
In one case description, a medial transchondral lesion was treated by retrograde drilling with a cancellous bone graft (72). The operation is recommended only when the cartilage overlying the defect is intact. The surgical approach was directed through the sinus tarsi into the talus. A guide pin was inserted under radiographic control through the body of the talus of the lesion at the dome of the talus. A bone biopsy needle was then advanced along the guide pin to the defect, and the proximal portion of the core of bone was used as the graft by reinserting it into the biopsy tunnel across the radiolucent fibrocartilaginous zone. Resolution of the radiolucent zone between the osteochondral fragment and the host bone of the talus developed, indicating successful healing. Obviously, this is a difficult approach and a more direct visualization may be preferable. Care needs to be taken when using the motorized drill to avoid thermal necrosis and penetration into the ankle. In a series of 11 cases of retrograde drilling, 27% had improved while 72% were unchanged at 1-year follow-up, whereas in 19 patients treated with transmalleolar drilling, 58% were unchanged and 42% had deteriorated (73).
Greenspoon and Rosman (74) reported a similar technique for the surgical treatment of medial transchondral lesions in children. The procedure consists of an osteotomy of the medial malleolus. Once the medial surface of the talus is exposed, a small ¼-inch osteotome is used to cut a window directly below the lesion and just above the insertion of the deep deltoid ligament. With a curette, the nonviable bone under the yellowed articular surface is removed. Cancellous bone graft from the tibial metaphysis is placed into the defect, and the medial malleolus is reduced and fixed. This procedure was performed on six children between 2 and 5 years of age with good results.
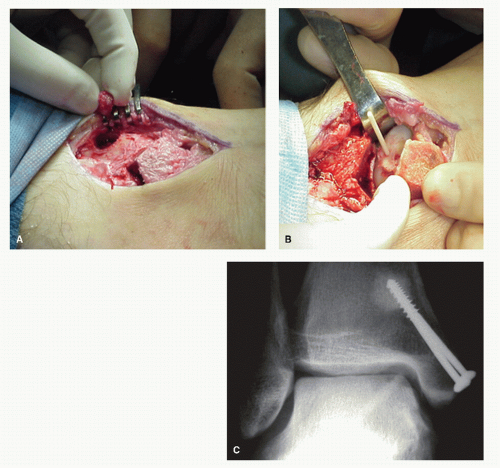
Ly and Fallat (37) have described another bone grafting procedure that was used in the treatment of larger symptomatic stage II and stage III lesions. If the articular cartilage is completely intact, an incision is placed in the cartilage directly over the osseous defect. The incision is circular, but a small section of the cartilage, approximately one-fourth of the circumference, is left intact as a point of stability. The cartilage flap is then retracted and any fibrous tissue is removed, while the hard sclerotic floor of the defect is curetted or trephined and drilled into the vascular trabecular bone. Cancellous bone is harvested from the distal tibial metaphysis utilizing a trephine and is packed tightly into the defect and compressed. The cartilage flap is reduced and fixated. Originally, cortical bone screws or 1.3-mm Orthosorb rods were used; now, either one or two allogenic cortical pins are used (Fig. 54.13).
Open reduction and internal fixation of stage IV transchondral fractures has not been described often. This is
probably because few stage IV fractures are diagnosed early enough after the injury so that open reduction can be considered. Accurate reduction may be difficult with a chronic lesion, owing to the fibrous tissue that forms on the cancellous bone (50).
probably because few stage IV fractures are diagnosed early enough after the injury so that open reduction can be considered. Accurate reduction may be difficult with a chronic lesion, owing to the fibrous tissue that forms on the cancellous bone (50).
Because of the intraarticular position and small size of the fragments, fixation can be challenging. Nash and Baker (75) reported a case of a stage III lateral lesion that was fixed using two 0.028-inch Kirschner wires (K-wires) with good results. Absorbable fixation would appear to be well suited for fixation of these types of fragments as well. Success has been reported with the arthroscope in fixing a stage IV lateral lesion with a 2-mm polyglycolic acid rod (76). Good results have been noted with fixation using 1.3-mm polydioxanone rods or 2-mm polyglycolide rods coated with polyactide and polydioxanone (77). Screws (1.5 or 2.0 mm) have also been successfully employed for the fixation of stage III and stage IV lesions with no evidence of subsequent loosening of the screws (Fig. 54.14).
Fibrin sealant, a physiologic glue containing freeze-dried human fibrinogen, bovine aprotinin, calcium chloride, and bovine thrombin has also been employed to fix stage III and
IV lesions. The fibrin is gradually decomposed and replaced by granulation tissue. The fibrin adhesive effect lasts for only 1 week, but the granulation tissue formed contributes to incorporation of the osteochondral fragment (78).
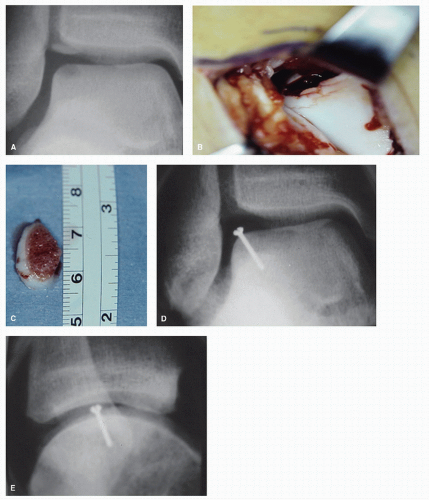
Meehan and Brage (79) have described using fresh osteochondral allografts for replacing large osteochondral lesions that are 10 mm or greater. The patients are first matched with a donor allograft on the basis of size only. A mediolateral measurement is taken 0.5 cm below the talar dome on the anteroposterior weight bearing ankle radiograph. A match is obtained when the size difference is 1 mm or less. The talar dome is exposed through an anterior approach between the tibialis anterior and extensor hallucis longus tendons for medial lesions and an anterolateral approach between extensor digitorum longus and peroneal tendons for lateral lesions. After debridement of osteophytes, an external fixator is then utilized to distract the ankle joint approximately 1 cm.
The recipient site is then prepared by first using a no. 15 blade to make a midline incision for a full-length sagittal cut through the talar dome. The medial or lateral portion of the talar dome is then removed utilizing a sagittal saw making
midline sagittal and horizontal cuts to remove the talar lesion. The horizontal cut is started at the interface of the articular cartilage and the talar neck. The hemitalus is then used as a template to prepare the donor graft with the same technique that was used to prepare the host site. The donor graft is then placed in the recipient ankle and the external fixator is then removed. The graft is fixated with two 3.0 partially threaded cannulated screws from dorsal to plantar (Fig. 54.15).
midline sagittal and horizontal cuts to remove the talar lesion. The horizontal cut is started at the interface of the articular cartilage and the talar neck. The hemitalus is then used as a template to prepare the donor graft with the same technique that was used to prepare the host site. The donor graft is then placed in the recipient ankle and the external fixator is then removed. The graft is fixated with two 3.0 partially threaded cannulated screws from dorsal to plantar (Fig. 54.15).
Hangody and Karpati (80) in 1990, developed a mosaicplasty technique, involving cartilage replacement in the knee with a series of cylinder grafts capped with cartilage and published his results in 1994. The mosaicplasty technique was
later applied to the talar dome osteochondral defects using ipsilateral cartilage grafts from the knee (81,82,83 and 84). Initially, the technique involved smaller plugs, which covered about 70% of the defect surface area (Fig. 54.16). The spaces between the hyaline-capped plugs later fill-in with fibrocartilage-like grout around tiles. Newer transfer systems involved somewhat larger plugs that can be interlocked and cover up to 100% of the surface defect (85,86).
later applied to the talar dome osteochondral defects using ipsilateral cartilage grafts from the knee (81,82,83 and 84). Initially, the technique involved smaller plugs, which covered about 70% of the defect surface area (Fig. 54.16). The spaces between the hyaline-capped plugs later fill-in with fibrocartilage-like grout around tiles. Newer transfer systems involved somewhat larger plugs that can be interlocked and cover up to 100% of the surface defect (85,86).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree