Osteochondral Lesions of The Talar Dome: Autologous Chondrocyte Implantation
Terence Y.P. Chin
Steve Mussett
Richard Ferkel
Mark Glazebrook
Johnny Tak-Choy Lau
Osteochondral lesions of the talus (OLT) are defects of the cartilaginous surface and underlying subchondral bone of the talar dome. In some instances, an associated subchondral cyst(s) forms subjacent to the OLT. The etiology of this condition remains uncertain although many follow a twisting injury to the ankle. In the absence of preceding trauma, a primary vascular insult is thought to be the cause. Patients with OLT report ankle pain, swelling, and mechanical symptoms of clicking or locking (1, 2).
OLT commonly occur on the anterolateral or posteromedial aspects on the talar dome. Trauma precedes up to 90% of anterolateral and 70% of posteromedial talar OLT (3). Loren and Ferkel (4) found a 61% incidence of traumatic talar articular surface lesions with acute ankle fractures. In addition, Hintermann et al (5) found an incidence of 79% cartilage lesions in 288 acute ankle fractures treated. Stufkens, Hintermann, and associates recently performed a long-term follow-up study of their consecutive prospective cohort of 288 ankle fractures, previously described above. One hundred and nine patients (47%) were available for follow-up at a mean of 12.9 years. Their findings showed that the initial cartilage damage seen arthroscopically following an ankle fracture was an independent predictor of the development of posttraumatic or osteoarthritis. They found lesions on the anterior and lateral aspects of the talus and on the medial malleolus correlated the highest with an unfavorable clinical outcome (6). OLT occur bilaterally 10% of the time (7).
Berndt and Harty’s (8) X-ray classification of OLT is still widely used. However, CT or MRI is essential to staging the lesion and guiding treatment (9, 10). The radiologic staging of OLTs is discussed in detail in the preceding chapters.
TREATMENT
Treatment of OLT depends on whether the lesion is acute or chronic, the radiologic stage and size of the lesion as well as the severity of the patient’s symptoms. This chapter will focus on the treatment of chronic OLT. Symptomatic high-grade lesions with significant detachment or displacement require surgery. Overall, nonoperative treatment has been shown to be ineffective in 25% to 45% of patients with symptomatic OLT (1, 11).
The aims of treatment are to reduce pain, restore ankle function, and protect the ankle joint from degenerative change. Ideally, this is achieved by restoring normal hyaline articular cartilage to cover the subchondral plate. If lower limb malalignment or ankle instability is present, these need to be addressed as well, particularly if any cartilage restoration procedure is contemplated.
Surgical options available for chronic OLT include the following:
Marrow stimulation: abrasion, drilling, or microfracture.
Cartilage restoration: osteochondral autologous transplantation system (OATS), osteochondral allograft transplantation, or chondrocyte implantation (e.g., autologous chondrocyte implantation [ACI]).
Loose and irreparable OLT must be removed. Marrow stimulation techniques produce nonhyaline fibrocartilage (predominantly type I collagen) with mechanical properties inferior to and likely less durable than native hyaline cartilage (12). Current treatment modalities that aim to restore hyaline or hyaline-like cartilage (consisting of mainly type II collagen) include OATS, osteochondral allograft transplantation, and ACI. OATS utilizes small hyaline cartilage covered bone plugs, harvested from the nonweight bearing areas of the knee joint to fill the OLT. Although good intermediate term results have been reported with OATS, concerns persist with regard to significant donor site morbidity, technical difficulties with the procedure, insufficient graft volume for larger lesions, chondrocyte death from plug impaction, difficulty with grafting “shoulder” lesions, mismatches of knee to ankle articular cartilage, and incomplete defect coverage (13, 14, 15 and 16). Osteochondral allografts have been used to treat mainly large OLTs, and acceptable results have been reported in several case series (level 4) (17, 18 and 19). Compared with autologous osteochondral grafts (harvested usually from nonweight bearing areas of the knee), talar allografts have the advantage of harvesting not only larger donor grafts, but also
grafts that are more anatomically congruent. With preoperative CT scans, the allograft can also be matched exactly to fit the recipient defect. The disadvantages of allografts, however, include the potential for disease transmission, fresh allograft availability, and longer healing times.
grafts that are more anatomically congruent. With preoperative CT scans, the allograft can also be matched exactly to fit the recipient defect. The disadvantages of allografts, however, include the potential for disease transmission, fresh allograft availability, and longer healing times.
OVERVIEW OF ACI
ACI technologies and techniques is a rapidly evolving field. A comprehensive review of this topic is beyond the scope of this chapter and the reader is referred to the references for further information (20, 21). Presently there are three generations of chondrocyte implantation techniques for osteochondral defects:
First-generation chondrocyte implantation involves the implantation of autologous chondrocytes under an autologous periosteal flap (classic ACI).
Second-generation chondrocyte implantation involves the implantation of autologous chondrocytes under a tissue-engineered collagen covering (e.g., Chondro-Gide and Bio-Gide, Geistlich Biomaterials, Wolhusen, Switzerland) or, more commonly, the implantation of a tissue-engineered scaffold seeded with autologous chondrocytes. The latter includes matrix-induced autologous chondrocyte implantation (MACI; Genzyme, Cambridge, MA) and Hyalograft C scaffold (Fida Advanced Biopolymers, Abano Terma, Italy).
Third-generation chondrocyte implantation essentially involves the creation ex vivo and implantation of a three-dimensional chondral grafts (21). Features of generation III techniques include the use allogeneic juvenile chondrocytes, novel chondroinductive and chondroconductive scaffolds, and specialized techniques to mechanically condition the developing chondral tissue ex vivo to enhance their material properties prior to implantation (22). Examples of these include the “Denovo” Engineered Tissue (ET) Graft (Zimmer, Warsaw, MO) and Neocart (Histogenics, Waltham, MA) (22).
Currently, only first-generation ACI techniques are in use in the United States for OLTs (23). Second-generation ACI is widely used in Europe and Australia, but are not currently approved by the FDA (24, 25). Third-generation chondrocyte implantation methods are in the early phases of human clinical trials in the United States. Others are not yet approved by the FDA for marketing (22).
FIRST-GENERATION ACI
First-generation ACI involves the implantation of previously harvested autologous chondrocytes that have been cultured and expanded in vitro. The implanted chondrocytes are secured to the defect by an overlying periosteal flap. Good results for the treatment of knee osteochondral lesions have been reported at 9- to 11-year follow-up (26). ACI’s success in the knee joint has led to its increasing use in the ankle as well as other joints.
ACI for OLT is performed in two stages. The first stage involves arthroscopically harvesting 200 to 300 mg of cartilage from nonweight bearing areas of the ipsilateral knee (edges of femoral condyles or intercondylar notch) or ankle. In the ankle joint, cartilage harvest has been described from the anterior edge of talar dome or tibial plafond, from the edges of the OLT during debridement or from removed loose fragment of OLT (27, 28 and 29). These cartilage specimens are then sent to the laboratory for chondrocyte isolation and proliferation (Genzyme, Cambridge, MA). A formal ankle arthroscopy is also performed to assess the size and depth of the OLT and whether bone grafting is required for large subchondral bony defects. In addition, associated pathology not accessible by future osteotomy is treated at the same time.
Details of chondrocyte isolation and culture are beyond the scope of this chapter and the reader is directed to the suggested reading list for more information (13, 30, 31). Briefly, the harvested cartilage is minced, washed with antibiotic solution, enzymatically digested, filtered, and centrifuged to obtain the chondrocytes. The chondrocytes are cultured to achieve a suspension consisting of 5 × 106 cells. The entire process requires 3 to 4 weeks.
The second stage is performed once the cultured chondrocytes are ready for implantation. In most cases, a lateral malleolar or medial malleolar osteotomy is required to access lateral and medial OLT, respectively (27, 29, 32, 33). The OLT is debrided to stable native cartilage at the rim of the lesion and to subchondral bone at the base. A flap of periosteum equal or slightly larger to the size of the defect is harvested from the distal tibial metaphysis and sutured to the defect with the cambium layer facing the subchondral bone using interrupted 5/0 Vicryl sutures. Fibrin glue is then used to seal the periphery of the flap, leaving a gap for the introduction of the chondrocytes. Normal saline is initially injected through this gap to confirm a water-tight seal and then removed. The cultured cells are then injected under the periosteal flap and the gap sealed with fibrin glue. The malleolar osteotomies are then internally fixed in the standard fashion (27, 29, 30, 32, 33 and 34).
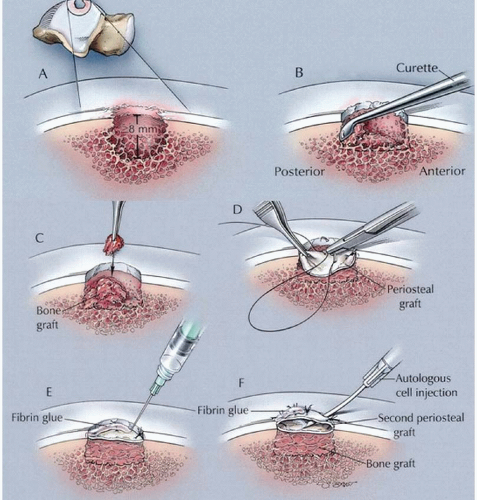
In some cases, a large cystic cavity underlies the OLT. This cavity has to be filled with cancellous bone graft and the level of the subchondral bone reconstituted prior to ACI. Some authors have implanted the autologous chondrocytes directly over the bone graft and sutured the periosteum over the defect (29, 30). Most, however, use the “sandwich technique” aimed at isolating the implanted chondrocytes from the underlying raw cancellous bone graft, thereby protecting the chondrocytes from unwanted bleeding and pluripotential cell contamination (27, 32, 33 and 34) (Fig. 90.1). After debridement and drilling of the base of the cyst, it is then filled with autogenous cancellous bone graft to the level of the subchondral plate.
A periosteal flap with the cambium layer facing away from the bone graft is secured to the OLT with sutures and fibrin glue. A second periosteal flap with the cambium layer facing into the initial periosteal flap (i.e., away from the joint surface) is then secured over the first with sutures and fibrin glue. The space between the two periosteal flaps represents an isolated chamber into which the chondrocytes are injected. The implanted chondrocytes are therefore “sandwiched” between the two periosteal flaps. The osteotomy is anatomically reduced with internal fixation.
A periosteal flap with the cambium layer facing away from the bone graft is secured to the OLT with sutures and fibrin glue. A second periosteal flap with the cambium layer facing into the initial periosteal flap (i.e., away from the joint surface) is then secured over the first with sutures and fibrin glue. The space between the two periosteal flaps represents an isolated chamber into which the chondrocytes are injected. The implanted chondrocytes are therefore “sandwiched” between the two periosteal flaps. The osteotomy is anatomically reduced with internal fixation.
EVIDENCE FOR FIRST-GENERATION ACI
A search of the English literature from 1994 (first report on ACI in humans by Brittberg et al. (13)) to August 2009 was performed in PubMed. Search terms included a combination of the following: “ankle,” “talar,” “osteochondral,” “osteochondritis dissecans,” and “chondrocyte implantation.” This revealed six studies on ACI for talar OLT (27, 29, 30, 32, 33 and 34). All were level 4 prospective case series with small numbers (largest study n = 14) and short to medium follow-up (2 to 5 years). Over 80% of patients
reported improvement in symptoms and high patient satisfaction. The vast majority of first ACIs was performed in patients who had failed previous marrow stimulation procedures.
reported improvement in symptoms and high patient satisfaction. The vast majority of first ACIs was performed in patients who had failed previous marrow stimulation procedures.
Peterson et al. (34) were the first investigators to perform ACI for osteochondral lesions of the knee and they have since published their early experience with OLT. They reported on 14 patients with ACI ± sandwich procedure (34). The average OLT size was 1.7 cm2 (range 0.3 to 3.5 cm2). Four had concomitant lateral ligament reconstruction. The results of this study are reported in more detail by Peterson in a separate publication (35). At an average follow-up of 32.4 months, 80% of patients reported improvement. Seven patients required repeat arthroscopy for periosteal hypertrophy and there was one patient with graft delamination.
In a level 4 prospective study, Whittaker et al. (33) utilized ACI in 10 patients with OLT. Two had the “sandwich” bone grafting procedure. The OLT had a mean area of 1.95 cm2 (1 to 4 cm2). Six of these had previous arthroscopic or open surgery. At an average follow-up of 23 months (range 12 to 54 months), the mean Mazur ankle score had improved from 51 pre-op to 71 post-op (P < .0005). Nine of 10 patients were pleased or extremely pleased with the result. Second-look arthroscopies were performed in nine patients at a mean of 13 months post-ACI. All OLTs had filled with macroscopically stable, but slightly softer and more irregular cartilage compared with the surrounding native cartilage. A full-thickness biopsies were performed in five patients showing hyaline-like cartilage in some regions in two patients and predominantly fibrocartilage in three patients.
Giannini et al. (30) reported similarly good results in a level 4 case series of eight patients treated with ACI as the primary procedure. The average OLT size was 3.3 cm2 (range 2.2 to 4.3 cm2) with average 26 months follow-up. One patient required bone grafting for a subchondral cyst, with the chondrocytes implanted directly over the cancellous bone graft and then covered with the periosteal flap (nonsandwich technique). One required a tibial osteotomy to correct malalignment. All patients were satisfied with the procedure, with AOFAS hindfoot scores improving from 32.1 to 91. Repeat arthroscopies in all eight patients showed complete filling of the defect with hyaline-type cartilage containing type II collagen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree