Osteochondral Autologous Transfer Procedure for Repairing Articular Cartilage Defects in the Talus: OATS Procedure
Mark E. Easley
Justin Orr
James A. Nunley II
INTRODUCTION
Management of symptomatic osteochondral lesions of the talus (OLTs) is challenging given the poor healing potential of articular cartilage and limited access to the ankle joint. OLTs may occur in any location on the talar dome and are typically observed centromedially or centrolaterally (1). In the absence of trauma, ossification defects, abnormal vasculature, emboli, or endocrine disorders may account for focal pathologic subchondral fractures of the talar dome. Acute OLTs are not always detected and are eventually managed as chronic lesions.
Not all OLTs are symptomatic; in fact, some are incidental findings. The specific reason OLTs produce symptoms is poorly understood (2). It is logical to assume that cartilage irregularities lead to intraarticular mechanical symptoms; however, patients rarely complain of sharp pain, locking, or catching. Most patients describe a deep ache within the ankle during weight bearing, probably due to repetitive high fluid pressure during cartilage loading that stimulates innervated subchondral bone deep to the cartilage defect. During cartilage loading, the compressed cartilage may force its fluid into microfractured subchondral bone, gradually leading to the creation of a subchondral cyst (2).
The standard of care in surgical treatment of subacute and chronic lesions is debridement of the OLT combined with drilling or microfracture to recruit mesenchymal stem cells that differentiate within the defect (3, 4 and 5). Curettage of the defect without drilling/microfracture
has also been described, but current recommendations favor maintaining the subchondral bone architecture when possible. Although favorable intermediate to long-term results have been reported, debridement/curettage and drilling/microfracture fill the void with fibrocartilage rather than restoring the physiologic hyaline cartilage surface (3, 4 and 5). Fibrocartilage is not considered as durable as the adjacent natural hyaline cartilage. Debridement and drilling/microfracture is effective in the vast majority of smaller diameter lesions (10 mm or less) but may be less successful in the treatment of many larger diameter osteochondral defects (10 mm or greater) (6). In smaller defects, debridement relieves mechanical symptoms, and the surrounding hyaline cartilage affords adequate mechanical support. With larger defects, the natural viscoelastic properties and mechanical support of the talar dome articular surface are compromised. Despite these limitations, debridement and drilling/microfracture remain the preferred primary surgical management even in larger OLTs (3, 4 and 5). These techniques are relatively straightforward, are inexpensive, and do not introduce donor site morbidity. Moreover, these techniques do not preclude a cartilage repair procedure at a later time.
has also been described, but current recommendations favor maintaining the subchondral bone architecture when possible. Although favorable intermediate to long-term results have been reported, debridement/curettage and drilling/microfracture fill the void with fibrocartilage rather than restoring the physiologic hyaline cartilage surface (3, 4 and 5). Fibrocartilage is not considered as durable as the adjacent natural hyaline cartilage. Debridement and drilling/microfracture is effective in the vast majority of smaller diameter lesions (10 mm or less) but may be less successful in the treatment of many larger diameter osteochondral defects (10 mm or greater) (6). In smaller defects, debridement relieves mechanical symptoms, and the surrounding hyaline cartilage affords adequate mechanical support. With larger defects, the natural viscoelastic properties and mechanical support of the talar dome articular surface are compromised. Despite these limitations, debridement and drilling/microfracture remain the preferred primary surgical management even in larger OLTs (3, 4 and 5). These techniques are relatively straightforward, are inexpensive, and do not introduce donor site morbidity. Moreover, these techniques do not preclude a cartilage repair procedure at a later time.
Debridement/drilling and microfracture tend to ineffectively relieve symptoms in (a) approximately 30% to 35% of OLTs, (b) OLTs associated with expansive subchondral cysts, and (c) larger OLTs (3, 4 and 5). Osteochondral autologous transfer system (OATS) serves to address OLTs unresponsive to or not amenable to arthroscopic debridement/drilling or microfracture, with the potential to restore hyaline cartilage in the OLT (3,4,7). Adequate exposure of the ankle joint to properly perform the osteochondral transfer may prove as challenging as the resurfacing procedure itself. Access to the OLTs is rarely the difficulty; instead it is typically the proper positioning of instrumentation that necessitates periarticular osteotomies and/or ligament releases.
The OATS procedure has been used extensively in the knee; over the past decade, this technique has been modified to address talar dome defects (3,7, 8, 9, 10, 11 and 12). The procedure comprises three basic steps: recipient site preparation (osteochondral plug removal at the OLT), harvest of a donor osteochondral graft from a non-weight-bearing portion of the ipsilateral knee (intercondylar notch or superolateral femoral condyle) or from an allograft talus size-matched to the patient’s affected talus, and transfer of the donor plug into the recipient site. Generally, there is no need to remove the osteochondral graft from the harvesting chisel; however, the surgeon may wish to do so in order to tailor the graft to accommodate unique recipient site graft requirements (4,7,11,12). We prefer not to disturb the donor graft during transfer. Options for graft sizes range from 6 to 10 mm, and multiple grafts may be harvested to resurface and physiologically contour defects exceeding 10 mm.
INDICATIONS AND CONTRAINDICATIONS
As noted above, OLTs may be incidental findings and not the source of the patient’s ankle pain. Evaluation should include diagnostic ankle injection with an anesthetic to confirm that the patient’s ankle pain is from an intraarticular source, with the OLT being the most likely. Operative treatment of OLTs is reserved for focal lesions that fail to respond to nonsurgical measures. Skeletally mature patients with unstable symptomatic lesions should not be subjected to a prolonged course of nonsurgical management given the low chance of spontaneous improvement (13). For OLTs with displaced fragments, strong consideration should be given to surgical management without a trial of nonoperative treatment (3,4).
The OATS procedure is indicated for management of symptomatic OLTs failing debridement/drilling or microfracture. OLTs associated with expansive subchondral cysts (stage V lesions) may represent an indication for primary surgical management with the OATS procedure. Larger OLTs, particularly those involving a shoulder of the talar dome, may require talar allograft reconstruction. In our experience, talar allografts may also serve as donor sites when utilizing the OATS procedure (14).
Contraindications to surgical management of OLTs include infection and medical comorbidities that preclude a surgical procedure. In addition, OLTs associated with diffuse ankle arthrosis are best managed with ankle arthrodesis or total ankle arthroplasty. Ankle malalignment and/or ankle instability represent contraindications, unless they are concomitantly corrected when the OLT is operated. The OLTs identified incidentally (i.e., ones that are not confirmed as the source of patient’s ankle symptoms) should not be prophylactically treated with surgical intervention.
Cartilage repair/reconstructive procedures, including the OATS procedure, for OLTs are contraindicated in patients with bipolar lesions (“kissing” lesions of the talar dome and tibial plafond) and diffuse (as opposed to focal) avascular necrosis (AVN) of the talar dome. Relative contraindications to the OATS procedure include patients with knee arthritis or patellofemoral symptoms, if the intention is to utilize the OATS instrumentation to harvest the osteochondral graft from the patient’s own knee.
PREOPERATIVE PLANNING
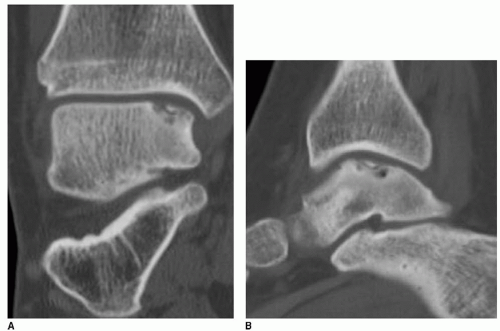
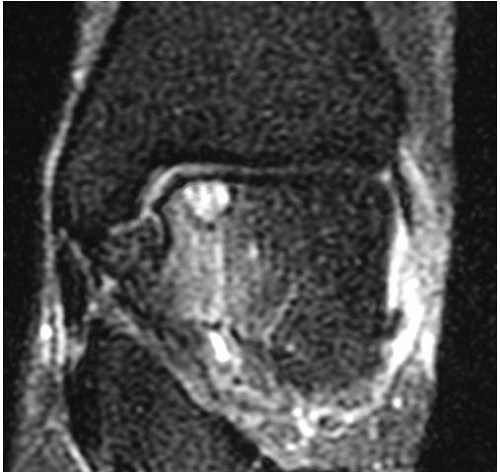
It is important to determine if the OLT is the source of pain prior to deciding on surgical management. When an OLT is observed, either on plain radiography (Fig. 42.1) or with magnetic resonance imaging (MRI) screening, workup must include diagnostic ankle injection. If pain does not resolve transiently with intraarticular anesthetic injection, the contribution of the OLT to the patient’s ankle pain is suspect. In the very least, intraarticular anesthetic injection should diminish the pain caused by the OLT, and residual pain should prompt further workup to identify associated ankle pathology. Once the OLT has been established as at least a contributing source of pain, we recommend further evaluation with a computed tomography (CT) scan (7). Even though MRI classifications have been accepted (6,15), CT scan evaluation most effectively isolates the OLT subchondral character (Fig. 42.2). Moreover, the CT scan best defines the dimensions of a subchondral cyst. MRI, with an ability to detect marrow edema associated with the OLT, typically suggests an exaggerated zone of involvement in the talar dome (Fig. 42.3). However, with higher quality MRI technology, details of the cartilage surface involvement can be determined (16,17). Associated ankle instability and/or malalignment must be defined, and the surgeon should plan to correct these pathologies in addition to addressing the OLT.
Staging of OLTs is based on the Berndt and Hardy’s radiographic classification: (I) small subchondral compression, (II) partial fragment detachment, (III) complete fragment detachment without displacement, and (VI) complete fragment detachment with displacement. More recent classification systems using CT and MRI have been described. Ferkel et al. (5) introduced a CT classification: (I) cystic lesion in talar dome with intact roof, (IIA) cystic lesion with communication to talar dome surface, (IIB) open articular surface lesion with overlying nondisplaced fragment, (III) nondisplaced lesion with lucency, and (IV) displaced fragment. Loomer et al. (18) added stage V to account for an OLT with a subchondral cyst. Hepple et al. (16) developed an MRI classification: (1) chondral injury only, (2a) cartilage injury with underlying fracture and surrounding bone edema, (2b) same as 2a without bone edema, (3) detached but undisplaced fragment, (4) detached and displaced fragment, and (5) subchondral cyst formation. Mintz et al. (17) have also described an MRI classification. MRI has also been utilized in follow-up to determine healing of OLTs (19, 20 and 21). Despite advances in imaging technology, the most effective staging tool probably remains arthroscopic inspection/probing of the OLT (5,22); however, in our opinion, arthroscopic inspection without detailed imaging studies may fail to provide an appreciation of an associated subchondral cyst.
Staging of OLTs, regardless of method, provides important details that impact treatment and prognosis. A purely subchondral lesion, with an intact cartilage surface, may be managed with retrograde drilling and bone grafting, a technique that does not violate the articular surface (23, 24, 25, 26 and 27). OLT associated with a subchondral cyst may respond less favorably to debridement and drilling when
compared to OLTs without subchondral cysts (28,29). Kumai et al. (28) suggested that poor results are to be expected in the management of OLTs associated with subchondral cysts, irrespective of treatment method. Robinson et al. (29) reported a 53% poor outcome in debridement/curettage and drilling of OLTs with subchondral cysts. Of note, Han et al. (6) suggested that OLTs associated with small subchondral cysts may be effectively treated arthroscopically.
compared to OLTs without subchondral cysts (28,29). Kumai et al. (28) suggested that poor results are to be expected in the management of OLTs associated with subchondral cysts, irrespective of treatment method. Robinson et al. (29) reported a 53% poor outcome in debridement/curettage and drilling of OLTs with subchondral cysts. Of note, Han et al. (6) suggested that OLTs associated with small subchondral cysts may be effectively treated arthroscopically.
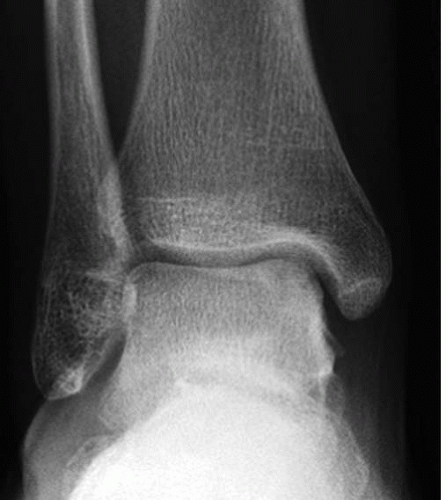 FIGURE 42.1 AP radiograph of the ankle suggests a medial talar dome defect, but it is not clearly defined. |
Plain radiographs of the ankle are necessary and typically identify complex OLTs; however, plain radiography is not a reliable method of detecting OLTs (see Fig. 42.1). MRI is a useful screening tool to identify OLTs and associated pathology (see Fig. 42.3). Applying the classification schemes noted above, OLTs may be characterized, particularly with respect to associated subchondral cysts. Occasionally, the signals related to adjacent marrow edema makes determination of the exact dimension of the OLT difficult, often creating an overestimation of its size. CT is most useful and essential in preoperative planning for the operative management of OLTs and most accurately defines the exact dimensions of the talar defect (see Fig. 42.2). Most OLTs to be managed by OATS have undergone prior arthroscopic management or inspection, and if available, we review prior arthroscopic images and/or video. Although arthroscopic detail is useful in assessing the articular surface defect, we also assess associated subchondral pathology with accompanying imaging studies.
With arthroscopic techniques or carefully placed arthrotomies, virtually every OLT may be accessed to perform debridement/drilling and/or microfracture techniques. In contrast, cartilage resurfacing/reconstructive procedures typically warrant ligament releases and/or periarticular osteotomies. The OATS instrumentation must be positioned perpendicular to the talar dome articular surface. Except for OLTs at the anterior or posterior margins of the talar dome (30), OATS frequently cannot be performed properly without medial malleolar osteotomy for centromedial OLTs and anterior talofibular ligament (ATFL) and calcaneofibular ligament (CFL) release and/or lateral malleolar osteotomy for centrolateral OLTs.
Muir et al. (31) provided guidelines for the surgical approach to resurface the talar dome, with an emphasis on perpendicular access. The authors concluded that much of the talar dome may be accessed without osteotomy but acknowledged that osteotomies are required to adequately expose larger OLTs. Even with medial or lateral malleolar or lateral tibial (Chaput) osteotomies, a residual central portion of the talar dome cannot be adequately exposed to allow perpendicular access (32). In a separate study also using a cadaver model, Garras et al. (33) confirmed these authors’ findings with regard to lateral OLTs. As observed in the cadaver model of Muir et al. (31), perpendicular access to the central talar dome is not possible via medial and lateral fibular osteotomies. Tochigi et al. (32) described a Chaput lateral tibial osteotomy, similar to a Tillaux fracture, to allow greater medial exposure to extensive lateral OLTs (32); Muir et al. (31) noted that this osteotomy still fails to allow access the central talar dome. The trap door osteotomy described by Sammarco et al., in which an anterior osteochondral wedge is removed from the distal tibia, may permit access to selected anterior central OLTs (10). Kreuz et al. (34) confirmed that the anterior tibial wedge osteotomy is effective in performing osteochondral grafting for posterior-central OLTs, without complications from the osteotomy. Although attractive, the osteotomy must be carefully planned to accommodate the instrumentation at the ideal location for perpendicular access, as coronal plane translation of the talus is not possible.
Ideally, an osteochondral graft should have an interference fit in the recipient site. To create this interference, harvesting chisels are 1 mm greater in diameter than their corresponding recipient site chisels. Perpendicular orientation of the grafts is essential; Koh et al. (35) have shown that eccentrically inserted grafts are subject to high contact stresses, shear stresses, and potential articular cartilage delamination. Two biomechanical cadaver studies demonstrate that osteochondral grafts should be inserted flush with the surrounding articular cartilage to optimize contact stresses (36,37). We strive to create proper orientation and a smooth transition between the native talar cartilage and the osteochondral graft without injuring the graft. We create recipient site to a depth of 11 to 12 mm and harvesting grafts 10 mm in length. Although this could lead to a propensity to countersink the graft, if performed carefully, this risk of potentially subjecting the articular component to excessive force during insertion is minimized. A graft that is too long for the prepared recipient site leads to undesirable excessive impaction or leaving the graft proud. Even with a perfect match of graft to recipient site and proper technique, Huntley et al. (38) demonstrated that heat generated from the chisels during harvest renders the peripheral third of the graft’s articular cartilage nonviable. Moreover, OLTs are frequently associated with sclerotic bone impenetrable by the standard chisels, prompting the use of reamers that potentially could create a rim of necrotic bone at the recipient site. We emphasize use of cold saline or sterile water irrigation if a reamer is employed to create the recipient site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree