Chapter 24 Osteochondral Allograft Transplantation
Articular cartilage defects present a challenging clinical problem for orthopedic surgeons. The limited healing capacity of cartilage has led to the development of various treatment options for symptomatic defects. Currently available techniques with at least midterm published outcome reports include marrow-stimulating techniques (subchondral drilling, microfracture), autologous osteochondral transfer system (OATS; mosaicplasty), autologous chondrocyte implantation, and osteochondral allograft transplantation. Each of these techniques has potential benefits and limitations. Resulting repair tissue and clinical outcomes may be variable with some of these techniques; as a consequence, the optimal treatment of many defects is controversial. Treatment algorithms have been proposed that take into account both patient and lesion factors, but minimal level 1 evidence exists to validate these algorithms.9,21,45,53
The use of fresh osteochondral allografts has a fairly extensive clinical history, extending over three decades.* Allograft transplantation is currently gaining in popularity owing to increasing appreciation that it reliably restores viable hyaline cartilage when compared with alternative treatment options for larger defects.7 Although logistic issues are associated with obtaining allografts, including waiting for an appropriate graft, the procedure itself is not very technically demanding in most cases. Fresh allografts are most useful in treating large chondral or osteochondral lesions (>2 cm), such as those seen with osteochondritis dissecans, trauma, osteonecrosis, and selected cases of degenerative arthrosis. Allografts can also be used as a salvage procedure after other cartilage resurfacing procedures are performed in young patients in whom arthroplasty options are undesirable.
Basic Science
The long-term success of osteochondral allografts is dependent on preservation of the hyaline cartilage surface, healing of the osseous base to the host bone, and maintenance of structural integrity during the remodeling process.66 Investigators have shown that chondrocyte viability is paramount in maintaining the normal extracellular architecture of hyaline cartilage and in preventing the development of degenerative joint disease, but the acceptable degree of chondrocyte viability required is unknown at this time.34,64 Although nonviable cartilage will appear grossly normal for a peroid of time, it will not maintain its histologic, biochemical, or biomechanical properties. As a result, the cartilage will fibrillate, develop clefts, and erode over time.34,64
Freezing of articular cartilage, although attractive in terms of decreasing immunogenicity and allowing storage, causes chondrocyte cell death to a variable degree with current preservation methods.63,70 Although up to 90% of isolated chondrocytes are able to survive the freezing process, freezing of chondrocytes embedded within their matrix has not been nearly as successful.62,63,70 This discrepancy is thought to result from poor penetration of cryopreservative, unequal rate of cooling, and high water content within the extracellular matrix.63,70 Because cryopreservation techniques have not been shown to preserve an acceptable degree of cartilage viability, fresh allografts are considered the mainstay for allograft transplantation of articular cartilage.22,27,61 However, whether or not the transplantation of fresh viable osteochondral grafts avoids a degenerative course superior to that of frozen grafts is a topic of ongoing controversy.34,71 It is important to note that current “fresh” allografts are actually refrigerated (not frozen) for a time prior to implantation, in contrast to historical fresh allografts, which were transplanted much closer to the time of procurement.41
Immune compatibility testing and postoperative immunosuppression are not required with osteochondral allograft transplantation despite the fact that both chondrocytes and subchondral bone have been shown to have immunogenic potential.38–4059 Chondrocytes are surrounded by a matrix that isolates them from the host immune cells and makes them relatively “immunologically privileged.”13,31 Although donor cells within the osseous component are immunogenic, their immunogenicity is muted and probably is not clinically significant in most patients because the cells are nonviable.15,23 When an osteochondral allograft is implanted into a host bed, a local inflammatory response is stimulated by both surgical trauma and the graft itself.67 This response is thought to peak between the second and third weeks and is primarily directed against the bone constituent of the graft that contains the marrow elements and other immunogenic elements.65 Therefore, pulse lavage of the bone just before implantation is recommended in an attempt to cleanse the graft and remove unbound antigenic elements. After the initial response, the inflammatory reaction may burn out or may persist for up to 18 months.17,67
In general, the osseous component of osteochondral allografts retains its structural integrity and is replaced with host bone via creeping substitution over a period of years.16,29,51,54 If the nonviable bony trabeculae cannot withstand mechanical stresses during the remodeling process, subchondral microfracture, collapse, and fragmentation may occur. Based on our understanding of the remodeling process and the stresses placed on grafts before completion of this process, it is somewhat surprising how relatively infrequently this occurs clinically. One potential benefit of minimizing the donor graft depth is a theoretical decrease in remodeling time.
Although cartilage matrix, glycosaminoglycan content, and biomechanical properties are not initially altered, percent chondrocyte viability, viable cell density, and metabolic activity have been found to progressively decrease over time following procurement.* Fresh osteochondral allografts harvested within 24 hours of donor death and preserved at 4° C have been shown to maintain up to 100% chondrocyte viability at 4 days.18,25,62 Contemporary allograft storage techniques preserve reasonably high chondrocyte viability with a significant decline in survival after 15 to 20 days.* By 44 days, chondrocyte viability falls to approximately 67%.57 Malinin et al showed in a primate model that osteoarticular allografts transplanted after 21 days of storage underwent more severe degenerative changes than allografts that had been stored for less than 21 days.44 Although increased cell viability is certainly optimal, acceptable chondrocyte viability appropriate for clinical implantation is controversial. This is due to reports of successful clinical outcomes and favorable histologic biopsy analysis of grafts implanted 4 to 6 weeks following procurement.24 Because of concerns related to infections, fresh osteoarticular allografts are currently stored hypothermically for a minimum of 14 days to allow serologic and microbiologic testing. After this time, grafts theoretically should be transplanted as soon as possible to optimize chondrocyte viability. Research continues regarding the optimal media composition for maximal preservation of chondrocyte viability while in cold storage.58,68
Long-term chondrocyte viability following osteochondral allograft transplantation has been shown in multiple biopsy and retrieval reports.† Researchers have biopsied transplants at various time intervals following the index procedure. Williams et al found 82% chondrocyte viability at 4-year follow-up with no significant immune reaction to the cartilage or the bone within the allograft.76 Davidson et al reported no significant detectable differences in graft versus native cartilage cell density or viability.24 Grafts were stored for 4 to 6 weeks before transplantation and were biopsied at a mean of 40 months postoperatively. Jamali et al confirmed that actual implanted donor chondrocytes were the source of cell viability by showing that female chondrocytes were still viable 29 years following transplantation into a male recipient.36 This potential for long-term survival supports the use of osteochondral allografts in an attempt to maintain extracellular matrix and thus prevent long-term articular degeneration within the graft.
Indications
At this time, it is unknown why some chondral or osteochondral lesions are asymptomatic, while others cause significant morbidity.73 Because we do not understand the natural history of most chondral lesions, surgery generally is indicated only for the treatment of symptomatic lesions. Debate continues regarding the treatment of asymptomatic full-thickness defects found at the time of surgery performed for other primary procedures (e.g., anterior cruciate ligament [ACL] reconstruction), but that discussion is beyond the scope of this chapter. The objectives of any biologic resurfacing procedure, including fresh allograft transplantation, are pain relief and functional improvement. Although our goal is the prevention of progressive joint deterioration and subsequent need for arthroplasty, most biologic resurfacing is performed in younger individuals with long life expectancies. Realistically, these biologic procedures should be considered a bridging procedure to improve pain and function until the patient is a more appropriate arthroplasty candidate.
Fresh osteochondral allografts are most useful in treating large chondral or osteochondral lesions (>2 cm), such as those seen with osteochondritis dissecans, trauma, osteonecrosis, and selected cases of degenerative arthrosis in young patients in whom arthroplasty options are undesirable. Allografts can also be used in a salvage procedure following failed cartilage resurfacing procedures. Patients with localized, unipolar, traumatic, nondegenerative, chondral lesions, osteochondritis dissecans, or osteonecrosis are believed to be optimal candidates for fresh osteochondral allografting and have obtained the best results.7,13,31 Relatively focal nonacute chondral lesions, which tend to be degenerative in nature, may also benefit from allografting procedures in the appropriate setting. However, results are often dependent on the status of the surrounding articular surface, and whether or not the underlying mechanical and physiologic factors that contributed to the initial chondral loss continue. Patients with associated pathology are more likely to benefit from a concomitant procedure, such as an unloading osteotomy or a meniscus transplant, in an effort to delay the progression of degeneration.
Primary treatment with an osteochondral allograft is often the optimal treatment when there is significant subchondral bone involvement of greater than 5 to 10 mm.13,21,53 The authors tend to favor osteochondral autograft or allograft for any lesion with subchondral bone involvement. Fresh osteochondral allografts are well suited for these types of lesions because they can restore the subchondral plate, in addition to hyaline cartilage.
Absolute contraindications to osteochondral allograft transplant include active infection and patients who are of appropriate age and activity level for prosthetic replacement. Associated grade III or IV kissing lesions of the tibiofemoral or patellofemoral articulation are generally considered a relative contraindication, unless both lesions are addressed at the time of the procedure.7 Malalignment, ligamentous instability, and meniscal insufficiency must be addressed before or at the time of the resurfacing procedure.31 Patients with inflammatory arthropathy, crystal-induced arthropathy, and synovitis are also relative contraindications. The effects of altered bone metabolism due to long-term steroid use or smoking have not been studied, although we generally consider this a contraindication.
Procurement, Screening, and Storage
Allograft tissue should be obtained from an accredited tissue bank that adheres to the guidelines established by the American Association of Tissue Banks (AATB) and the U.S. Federal Drug Administration (FDA).2,72 Graft procurement should be carried out within 24 hours of death under strict aseptic conditions.4,6,32 Donors are screened for risk of disease transmission, including detailed medical and social history for risk factors of exposure to human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.3,72 The FDA had previously mandated titers for HIV-1 and HIV-2 antibody, hepatitis B surface antigen, hepatitis core antibody, hepatitis C antibody, human T-lymphocyte virus 1 antibody, HIV p24 antigen, and the rapid plasma reagin test for syphilis. Beginning in 2005, all AATB-accredited tissue banks were required to perform nucleic acid testing (NAT) for both HIV-1 and the hepatitis C virus to further reduce the window period associated with conventional (enzyme immunoassay [EIA] method) donor screening antibody or antigen tests.10
One of the issues of greatest concern surrounding the use of fresh allograft material is the risk of infectious organism transmission, including viral and bacterial disease. Current tissue bank processing and donor screening reduce the risk of disease transmission to very low levels, although the exact risk estimate is unknown for fresh osteochondral allografts.3,10–12,69 Not all cases are detected, and surveillance systems have not been designed to define the true incidence of these infections. In 1995, based on reports in the literature, the incidence of infection was estimated to be 0.02% from approximately 20,000 organ transplants per year, and 0.0004% from approximately 900,000 allografts per year.19 In 2007, AATB-accredited tissue banks distributed just over 1.1 million musculoskeletal allografts. Most of these were processed grafts, which went through proprietary washing and sterilization. Only approximately 2200 of the distributed grafts were classified as osteochondral allografts.10 Heightened awareness among clinicians and improved diagnostic tests have enhanced the detection of tissue-associated infection.
The risk of bacterial transmission from contaminated fresh allograft tissue is of greater concern compared with processed nonviable grafts because cytotoxic cleansing treatments cannot be utilized. One case report describes a fatal bacterial infection following implantation of a fresh osteochondral allograft contaminated with Clostridium.37 A subsequent investigation by the Centers for Disease Control and Prevention identified 26 potential patients with allograft-associated infection: 13 with Clostridium species and 14 associated with a single processing agency.37 Malinin et al found that 64 of 795 consecutive donors of musculoskeletal tissue (8.1%) had Clostridium contamination.43 They also found that the risk of Clostridium contamination increased with the length of time between donor death and allograft harvest. In addition to a review of the medical records, cultures of tissue, donor blood, and donor blood marrow are used as screening tools to limit the risk of transmitted bacterial infection.46,47 Episodes of allograft-associated infection in the past decade have improved awareness, enhanced our understanding of the problem, and may decrease this risk in the future.
Preoperative Planning
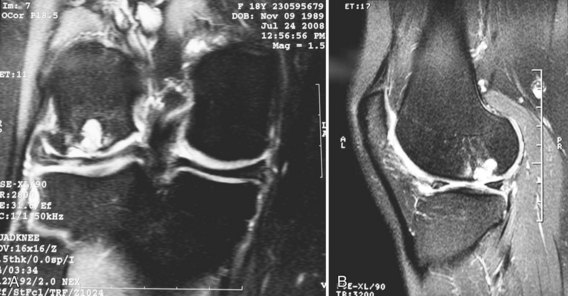
Confirming that the patient is an appropriate candidate for a fresh allograft is important before tissue is ordered. This often can be accomplished with high-quality magnetic resonance imaging (MRI), in addition to weight-bearing radiography (Fig. 24-1A and B). When concern arises about whether a patient is a candidate for an allograft, we typically perform a diagnostic arthroscopy to evaluate the lesion of interest, as well as the remainder of the knee. If the patient had a recent arthroscopy performed elsewhere, arthroscopic pictures, operative reports, and radiographic studies are often sufficient. Long-alignment films are required to determine whether associated malalignment necessitates a concurrent unloading osteotomy.
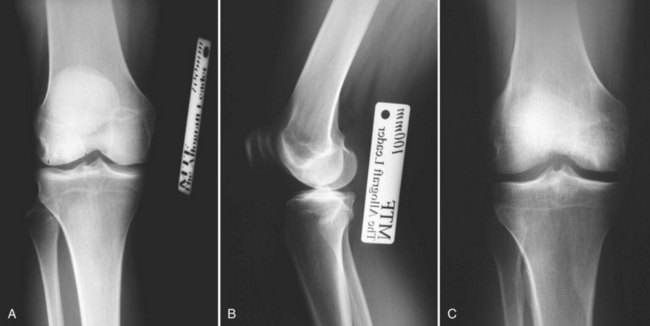
Obtaining an acceptable donor graft from an AATB-accredited tissue bank is a key component of the procedure. Size matching from an appropriately screened donor is the main criterion for obtaining an acceptable osteochondral graft. Graft size is measured radiographically. Anteroposterior and lateral radiographs with magnification markers placed at the patient’s joint can be used to calculate dimensions (Fig. 24-2A through C). Care must be taken to allow for appropriate correction of magnification error. A match is considered acceptable at ±2 mm; however, significant variability in anatomic morphology may be noted. Differences between men and women may be particularly apparent. Larger defects typically require more precise graft matches to optimize articular congruity. However, when an osteoarticular plug or dowel technique (mega-OATS [osteochondral autograft transfer system]) is used, a same-size or larger donor surface is often acceptable. Accepting a smaller donor for a large graft is not recommended. Wait times for an acceptable graft vary and can be both lengthy and frustrating for patients. It is common to send measurements to several trusted AATB-certified tissue banks in an effort to increase the odds for procuring a compatible graft within a reasonable time frame.
Surgical Technique
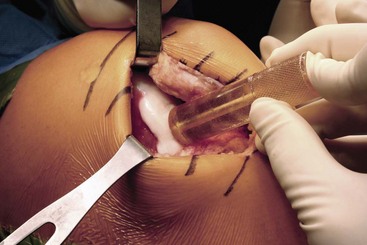
Great care is taken when entering the joint to avoid injuring the articular cartilage or the anterior horn of the meniscus. In cases where the lesion is posterior or very large, the meniscus may need to be taken down at the meniscocapsular junction and may be repaired as part of the closure. Larger bulk allografts may require more extensive exposure. For a typical condylar lesion, retractors are placed medially and laterally, including one in the notch, to retract the patella and gain exposure to the joint (Fig. 24-3). The knee is then flexed or extended until the lesion is exposed through the arthrotomy site. Once visualization is achieved, the lesion is inspected and palpated to determine its depth, size, and margins.
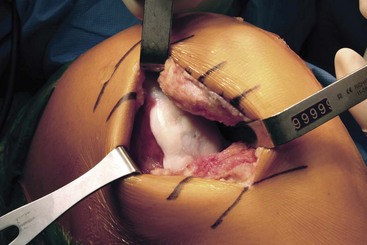
Figure 24-3 Exposure of osteochondral lesion of the lateral femoral condyle through lateral parapatellar arthrotomy.
Press-Fit Plug Allograft Technique
Several proprietary instrumentation systems are currently available for the preparation and implantation of press-fit plug allografts between 15 and 35 mm in diameter. Although we describe only one of the instrumentation systems in our technique, most systems are similar. The size of the proposed graft is determined by using a sizing dowel to cover as much of the defect as possible while limiting the sacrifice of normal tissue (Fig. 24-4). Sometimes a lesion is more amenable to the use of two overlapping cylindrical plugs (termed “snowman” or “mastercard” configuration). Once the sizing dowel is in the appropriate position, a guide wire is driven several centimeters into the center of the lesion (Fig. 24-5). It is important that the guide wire be drilled perpendicular to the curvature of the articular surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree