Chapter 32 Orthoses for persons with postpolio sequelae
Several theories exist on the cause of the new muscle weakness and atrophy commonly observed in this patient population. First, many of these individuals are aging and consequently have a significantly diminished neuromuscular system. Small losses that typically occur with normal aging may appear more pronounced because they represent loss of a greater percentage of the surviving nerve and muscle cells after poliomyelitis infection. Second, the neuronal sprouting and neuroplasticity that occurred during the acute recovery period may be more physiologically fragile and, therefore, place the patients at risk for premature aging and breakdown. In addition, the neuronal sprouting that occurred caused the development of giant motor units. The metabolic demand of these giant motor units is tremendous, as one cell body now is innervating many times more muscle cells than it had previously. If the physiological demand is too great for too long a time period, some of the neuronal sprouting will be pruned. There is some evidence of instability at the neuromuscular junction, which may cause muscular fatigue and less endurance.35 Additionally, patients with muscle pain due to overuse may show elevated creatine kinase levels in their blood, indicating damage of the skeletal muscle at a cellular level.9
Historical perspective
Acute anterior poliomyelitis, also known as infantile paralysis, is a devastating infectious disease. After smallpox, it may become the second infectious disease completely eradicated from the planet. The infectious agent is the polio virus, of which there are three types: I, II, and III. The name poliomyelitis is derived from the Greek word polio (meaning gray) and myelitis (meaning infectious process of the spinal cord). As the prefix polio indicates, infection of the spinal cord occurs exclusively in its gray matter, which is populated by neurons. However, the polio virus also may affect the motor neurons of the cranial nerves, which are located in the brain.
The first written description of a patient with a clinical picture resembling poliomyelitis is credited to Salzmann, who in 1734 reported on the history of a person who had contracted a paralytic condition during childhood but had a gradual recovery and partial cure. The patient died as an adult after 10 years of being psychologically upset, becoming an alcoholic and speaking of being tired of life and desiring to die. Postmortem examination of the body revealed “degenerated muscle bellies replaced by fatty and fibrous tissue.”32 If this person indeed had poliomyelitis, were his problems before death manifestations of what we now call postpolio syndrome? In 1789, Underwood described a case of “debility in the lower extremities,” but whether the patient’s weakness was actually due to another cause, perhaps a vertebral lesion due to tuberculosis (Fishbein et al., 1951), has been questioned.10 Other extensive and creditable descriptions were made in the 19th century by the German physician Jacob Von Heine (1806–1879) and the Swedish physician Oskar Medin (1847–1928). For about a century, throughout Europe the disease had been referred to as the Heine-Medin disease rather than poliomyelitis because the simple mention of the word poliomyelitis created a lot of panic in the general population, especially during the frequent summer epidemics.
The medical literature of the late 1800s contains at least three reports of patients who presented problems similar to those of our current PPS cases.6,8,31 In the period from 1875 to 1975, approximately 200 cases were reported. However, the problem did not become readily apparent in the United States until the early 1980s, when numerous persons who had been affected with poliomyelitis during the epidemics of the 1930s, 1940s, and 1950s began to develop clear manifestations of PPS. It has been estimated that approximately 30% of the more than 1.5 million polio survivors in the United States are affected with the syndrome, but the real prevalence among survivors likely is much greater.
Use of orthoses for persons with PPP proliferated considerably in the first half of the 20th century in the United States and in Europe. The prescription of orthoses for polio survivors required elaborate adaptations to each patient’s needs given the scattered and asymmetrical paralysis typical of poliomyelitis. Use of off-the-shelf orthoses rarely was possible. The availability of new materials that are lighter than the traditional leather and steel used in older orthoses has made possible better customization of the newer devices described in this chapter, which are much more accepted by polio survivors than the older and heavier devices.
Many individuals who had recovered from the initial polio infection began to experience increased weakness in muscles known and not known to have sustained polio-related damage and presented for medical help in the 1970s and 1980s.35 PPS has been described as a syndrome since the mid 1980s. From 25% to 70% of all polio survivors develop PPS. The median reported time from acute poliomyelitis to PPS is 35 years.34
Current research
Advances in technology have created more options in orthotic design and fabrication that adequately protect and assist paretic or paralyzed lower extremities in gait, standing, and transfers. The most commonly seen advance in orthotic fabrication is the use of plastic or carbon fiber materials. These materials act primarily to decrease the weight of the orthosis, but they also increase the level of mechanical control and/or support of the limb. Decreasing the weight by even a few grams or ounces can make a huge difference for a person with weakness. Patients should select the lightest-weight shoes they can find to decrease the overall weight and energy needed to move the affected limb. Excessive hip flexion during limb advancement is a frequently observed compensation. Increased weight on the limb, particularly distally, can greatly impact the person’s ability to adequately clear the foot in swing.13,27,35
One of the technological advances in the area of treating PPS patients is the lightweight carbon fiber knee–ankle–foot orthosis (KAFO). Orthotists fabricate the orthosis with the lightest-weight materials available to increase patient acceptance. In a study of 14 patients with PPS and 14 age- and gender-matched healthy subjects, polio survivors had 28% lower walking speed, 9% higher energy consumption, and 40% higher energy cost.4 Lower extremity muscle strength was significantly correlated with walking speed and energy cost. Five of the subjects wore unilateral KAFOs, one wore a unilateral ankle–foot orthosis (AFO), one wore bilateral AFOs, and six used canes with or without their orthoses.4
Heim et al.13 conducted a pilot study examining the efficiency of lightweight carbon fiber KAFOs in the management of symptoms of people with PPS. Thirty subjects with PPS who wore metal and leather KAFOs participated in the study; 27 completed the study. Twenty-one of the 27 wore their custom-made carbon fiber KAFOs daily and reported that the devices were lighter weight, better fitting, and more aesthetic. The carbon fiber KAFOs weighed an average of 1,150 g compared to 1,720 g for the metal KAFOs (approximately one third heavier in weight). Eight of the 27 participants preferred the metal orthoses because of skin irritation, excessive sweating, and the inability to change the shape of the device after fabrication to accommodate weight gain or swelling in the carbon fiber orthosis. The participants reported other disadvantages of the carbon fiber orthoses, such as the exact fit required, greater manufacturing time (average 17.5 weeks), and greater expense.13
Another technological advance is the use of a stance control knee joint within KAFOs. These electronic or mechanical knee joints allow automatic locking during stance phase and automatic unlocking for knee flexion during swing phase.12,17 Hebert and Liggins12 performed a case study of a 61-year-old patient with PPS, comparing various gait parameters and the Physiological Cost Index (PCI) with use of a KAFO in either the stance control or locked mode. No significant changes were noted in velocity, cadence, stride length, or step length, or during toe-off. With the KAFO in locked knee position, the knee remained in approximately 6 degrees of flexion throughout all phases of gait. With the KAFO in stance control mode, the knee was in 17 degrees of flexion and progressed to 55 degrees of flexion at 75% of the gait cycle. Stance control mode decreased transverse plane pelvic rotation by 6 degrees and overall excursion through the entire gait cycle by 6 degrees. In stance control mode, vertical pelvic displacement was reduced from 67.0 ± 5.9 mm to 58.7 ± 5.6 mm. Lateral pelvic excursion also was reduced, from 128.8 ± 12.0 mm to 108.6 ± 11.0 mm. PCI was 0.447 at self-selected walking velocity of 49.2 m/min in stance control mode compared to 0.554 at a velocity of 49.7 m/min with a locked knee.12
Similarly, a pilot study was conducted of two participants with PPS and one with an incomplete spinal cord injury (SCI). All subjects demonstrated improved velocity, cadence, stride length, and step length while using the KAFO in stance control mode. Less hip hiking and lateral trunk flexion were observed. The two participants with PPS completed the obstacle course more quickly while using stance control. While walking for 5 minutes on a treadmill, the two participants with PPS had lower increases in heart rate in stance control than locked condition, and the participant with incomplete SCI experienced a lower treadmill-induced heart rate while using the stance control mode.23
Waring et al.36 examined the effects of lower extremity orthotic management on gait, pain, and fatigue in individuals with PPS. Among 104 participants, they found that a new orthosis was more frequently prescribed for a previously braced limb or an additional device for a previously untreated limb for patients who had worn orthoses earlier in their lives than for those who had never worn orthoses (28/56 who had worn orthoses vs 9/48 who had not worn orthoses). Seventy-two percent of those who had undergone an ankle fusion required new orthoses. A follow-up questionnaire returned by 78% of participants showed that appropriate orthoses significantly improved fatigue, weakness, walking ability, perceived walking safety, and knee pain.36
The frequency and types of orthoses prescribed for individuals with postpolio sequelae were described in a retrospective study of 5,045 cases of postpolio infantile paralysis conducted in Saudi Arabia. A total of 3,280 lower extremity orthoses were supplied, including 730 AFOs, 1,518 KAFOs, and 1,032 hip–knee–ankle–foot orthoses (HKAFOs) or bilateral HKAFOs.1 The number of individuals in this study who were polio survivors diagnosed with PPS is unclear.
In a retrospective study of 772 patients diagnosed with PPS seen at the in-house postpolio outpatient clinic between 1999 and 2005 at the Postpolio Outpatient Clinic at the Texas Institute for Rehabilitation and Research (TIRR), 337 either wore orthoses or were prescribed new orthoses. A total of 207 KAFOs (51.8%) and 191 AFOs (47.8%) either were worn by or prescribed for these patients. Of the KAFOs, 78 had been used for the patient’s lifetime since onset of acute poliomyelitis (most with locked knees), 52 orthoses with posterior offset knee joints (one replacing lifetime locked knee joint) had been prescribed, 24 orthoses with stance control knee joints (two replacing lifetime-use locked knee joints) had been prescribed, and the rest had locked knees with drop/ring, bail, or cable release locks, or undocumented types of knee joints. Of the AFOs, 71 were rear entry ground reaction design with articulating ankle joint and dorsiflexion assist; 17 were rear entry ground reaction design with either solid or undocumented ankle joints; 29 were traditional solid ankle; 29 were posterior leaf spring design; eight were traditional articulating ankle with dorsiflexion assist, plantarflexion stop, or both; 32 were undocumented ankle joint; and three were supramalleolar design. Additionally, one Swedish knee cage and one knee brace designed to unload the femorotibial joint to slow joint deterioration were noted. Fifty-seven individuals (16.9%) wore bilateral devices.18
Orthotic management
Patient evaluation
Management of patients with postpolio sequelae requires a thorough history and clinical evaluation by the orthotist. The history should include a review of the patient’s past orthotic management, surgical interventions, existing painful joints, vocational and nonvocational activities, along with the patient’s description of how often he or she is falling or nearly falling and the circumstances surrounding those falls or near falls. Clinical evaluation should include assessment of basic manual muscle strength and passive range of motion, static alignment of the joints of the lower limb, overall posture of the patient, and careful observational gait analysis.26
Through the course of this initial evaluation, the orthotist must assess the patient’s willingness to accept the device. Often patients with PPS have managed quite well without an orthosis for the majority of their lifetimes and are reluctant to use an orthotic device. Many perceive that use of an orthotic device signifies that a disease they had overcome has again reared its head and begun to control their lives.34 Finally, it is important that the orthotist ask the patient what he or she hopes the device will do for him or her. If the patient’s expectations are unrealistic, the orthotist must carefully define what the device can and cannot reasonably be expected to do for the patient. Unrealistic expectations can lead to disappointment that in turn leads the patient to dissatisfaction with or even abandonment of the device. With this information and the team’s prescription for the orthotic design, the orthotist can begin the process of designing the optimal orthosis.
Design
Ankle–foot orthoses
An AFO can be used to stabilize and protect the joints of the foot and ankle and provide swing phase clearance and stance phase stability. Secondarily, an AFO will have an impact on knee kinematics.21,24,33 These devices are available in a variety of designs. The designs most commonly used in the management of the population with postpolio sequelae are discussed here.
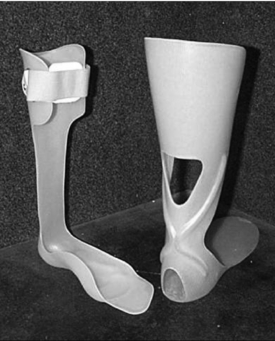
Posterior leaf spring AFO
The posterior leaf spring ankle–foot orthosis (PLS AFO) provides clearance of the foot through swing phase (Fig. 32-1). Its impact in stance phase is limited to mild control of ankle inversion or eversion and only mild resistance to tibial advancement. It permits smooth advancement from initial contact to loading response and eliminates “foot slap.” As with the metal Klenzak dorsiflexion assist AFO (described below), this device is appropriate only for a narrow range of individuals with PPS and should be used only when good stance phase stability exists.33
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree