Abstract
Sports medicine physicians often treat athletes in pain with non-steroidal anti-inflammatory drugs (NSAIDs). However, there is a lack of high-quality evidence to guide NSAID use. Their adverse effects have clinical relevance, and their possible negative consequences on the long-term healing process are slowly becoming more obvious. This article provides some practical management guidelines for the use of NSAIDs, developed to help sports medicine physicians deal with frequent sports-related injuries. We do not recommend their use for muscle injuries, bone fractures (also stress fractures) or chronic tendinopathy. In all cases, if chosen, NSAID treatments should always be kept as short as possible and should take into account the specific type of injury, the level of dysfunction and pain.
Résumé
L’épisode douloureux en médecine du sport est fréquemment traité par des anti-inflammatoires non stéroïdiens. Pourtant, à l’heure actuelle, une controverse se fait jour quant à leur efficacité et leurs effets secondaires ne sont pas anodins. L’inhibition de la réponse inflammatoire précoce peut altérer la cicatrisation naturelle d’une lésion et avoir un impact négatif sur le processus de réparation ultérieur. Dans le cas de lésions ligamentaires fraîches, l’administration d’anti-inflammatoires non stéroïdiens paraît être une option utile, à condition de respecter une cure de courte durée. Lors de tendinopathie par surcharge, il n’y a pas à proprement parler de phénomène inflammatoire ; de fait, les anti-inflammatoires non stéroïdiens ne sont pas recommandés. Il convient aussi de les éviter après une fracture en raison de leurs effets délétères sur la formation osseuse. Enfin, les études ne démontrent pas d’intérêt notable à leur utilisation lors de lésions musculaires aiguës. Une réflexion s’impose donc pour savoir s’il est justifié de masquer les symptômes douloureux d’un athlète afin de lui permettre une interruption la plus courte possible de sa pratique sportive, au détriment peut-être de sa guérison.
1
English version
1.1
Introduction
The supposed best method for the immediate treatment of most sports injuries is well known: rest, ice, compression and elevation (RICE protocol). However, although it seems to make sense to continue recommending the use of this protocol, no study has really proved its advantages .
In order to reduce traumatic or post-traumatic pain, the current practice is to prescribe analgesics or non-steroidal anti-inflammatory drugs (NSAIDs). However, NSAIDs are not without side effects and, despite these side effects, some NSAIDs are sold as over-the-counter drugs, or at least are accessible without strict medical control.
For various reasons, athletes regularly take NSAIDs in order to continue their athletic activities, despite acute traumatic injuries or overload injuries. They also want to accelerate their return to the playing field after an injury. They even take NSAIDs as a preventive measure. For example, during the 2000 Olympic Games in Sydney, Canadian athletes used NSAIDs more than any other medication . Similarly, a survey of American football players showed that one out of seven high school athletes took NSAIDs daily and the 29% of college athletes took them as a preventive measure on the day of a game . Warner et al. found a similar occurrence in their study of athletes: independent of their analgesic effect, the athletes mentioned a potential performance improvement to justify taking these medications .
For a sizeable number of practitioners, the medical knowledge about the harmful consequences of regular use of NSAIDs is limited to gastrointestinal (GI) disorders and renal function. However, recent medical literature has shown that the harmful effects of NSAIDs extend to cell metabolism and growth of the primary tissues making up the musculoskeletal system . These recent developments raise an “ethical” question for clinical practice: should medical practitioners only take into account the short-term analgesic effect of NSAIDs in order to encourage immediate performance improvements, or would it be better to stress the potential harmful long-term consequences of their use?
This article doesn’t attempt an exhaustive analysis of the literature on this subject. Rather, it provides an update destined to help primary care physicians, general practitioners and sports medicine specialists to better evaluate the risks and benefits inherent to the occasional or regular use of NSAIDs for athletes, whether the injury be to the ligament, tendon, bone or muscle.
To create this update, we used the traditional data sources, Pubmed and Embase, from 1997 to 2008, with the following keywords: non-steroidal anti-inflammatory, musculoskeletal system, tendinopathy, bone fractures, ligament injuries and muscle tears. We then each individually read all the article titles and abstracts and selected those that dealt with the role of NSAIDs in the motor system pathologies encountered frequently in sports medicine. We chose a total of 55 articles appearing in peer-reviewed journals, either literature reviews, meta-analyses or random controlled studies. We didn’t attempt to classify these articles with respect to their methodological quality.
1.2
Non-steroidal anti-inflammatory drugs: general information
1.2.1
Mechanisms of action
NSAIDs are most frequently administered orally; this implies that, depending on their individual properties, NSAIDs are absorbed by the digestive system, enter the bloodstream, and are metabolized by the liver or the kidneys. NSAIDs can also be administered topically or via intramuscular injection. Table 1 provides a synthetic classification of the most common NSAIDs, according to their plasma half-life.
| Short Half-life | < 6 h | Long half-life | > 6 h |
|---|---|---|---|
| Aspirin | 25–33′ | Diflunisal | 8–12 |
| Diclofenac | 1–2 | Naproxen | 12–15 |
| Ibuprofen | 1–2.5 | Salsalate | 3.5–16 |
| Ketoprofen | 1.5–4 | Sulindac | 16–18 |
| Fenoprofen | 2–3 | Piroxicam | 24–38 |
| Mefanamic acid | 2–4 | Nabumetone | 24 |
| Meclofenamate | 3–4 | Oxaprozin | 25 |
| Indomethacin | 4–5 | Phenylbutazone | 77 |
| Flurbiprofen | 4–6 | ||
| Ketorolac | 4–6 | ||
| Etodolac | 6–7 |
As Fig. 1 shows, NSAIDs work by blocking cyclo-oxygenase (Cox), thus inhibiting the synthesis of prostaglandins from arachidonic acid, while one part of the arachidonic acid cascade continues on the lipo-oxygenase pathway. Migration, aggregation and neutrophile and macrophage functions are also inhibited. Two Cox iso-enzymes (which probably have sub-classes) have been identified: a constitutive form called Cox-1 and an inducible form called Cox-2. Cox-2 is overexpressed locally in cases of inflammation, and the prostaglandin synthesis that results from this overexpression supports the lesional process. Traditional NSAIDs inhibit the two iso-forms, which effectively reduces the inflammatory response, but also reduces gastric protections and interferes with renal function. Selective Cox-2 inhibitors have been introduced to reduce these side effects, especially the gastric ones.
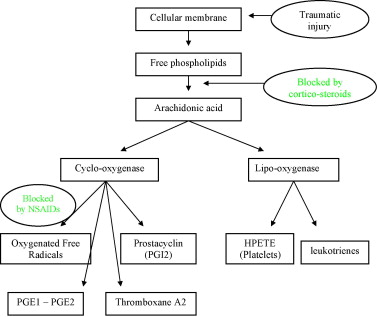
In the light of these observations, it seems more and more obvious that NSAIDs, because they inhibit the initial inflammatory responses, can have a negative impact on the repair process because they alter the natural healing process of an injury.
1.2.2
Adverse effects
The adverse effects of all types of NSAIDs are related to the GL, the cardiovascular systems, the kidneys and the liver. GL side effects (i.e., dyspepsia, nausea, ulcers and bleeding) appear primarily when NSAIDs are taken frequently. However, a much higher relative risk (RR) of bleeding in the upper GL tract than that of control subjects appears after only 1 month of regular NSAID doses. The RR is 4.0 for such traditional NSAIDs as diclofenac and naproxen, 3.0 for diclofenac taken with misoprostol, 1.9 for rofecoxib and 1.0 for celecoxib . As a preventive measure, it is thus recommended to limit the length of administration, to take NSAIDs with meals and/or to prescribe a proton pump inhibitor or to associate diclofenac and misoprostol.
To reduce the adverse effects on GL system, selective Cox-2 inhibitors were introduced in 1999. However, the studies published after their introduction pointed out that these substances disturb the prostacyclin–thromboxane balance, which is essential for maintaining vascular homeostasis . Disturbing this balance increases the risk of thrombosis . One of these selective Cox-2 inhibitors, rofecoxib, was withdrawn from the market because of an increased pro-thrombotic effect that was responsible for a high number of vascular accidents. In Switzerland, another one, valdecoxib, was also withdrawn from the market for safety reasons. To our knowledge, only celecoxib was kept on the market. If the RR of myocardial infarction is evaluated, the RR of rofecoxib is 1.35 with a dose-dependant effect (over 25 mg/day, the RR rises to 2.19), while celecoxib has an RR of only 1.06. In addition, high RR values have also been found for certain non-selective NSAIDs, such as diclofenac and indomethacin, which have respective RR values of 1.40 and 1.30 .
In addition, there is a risk of post-traumatic hemorrhage in athletes involved in contact sports or “at-risk” sports . A less frequent side effect, renal failure, has been observed in elderly subjects but also in dehydrated subjects. Since dehydration happens frequently to athletes when playing sports, this should be kept in mind.
1.3
Non-steroidal anti-inflammatory drugs: parenteral forms
1.3.1
Topical applications
Large-scale clinical studies about topical anti-inflammatories are rare, thus their conclusions are obviously incomplete but interesting nonetheless. Topical NSAIDs appear to dispense sub-cutaneous therapeutic doses through tissue penetration, while reducing the adverse systemic effects by lowering the rate of circulation of the substance in the bloodstream . A meta-analysis of diclofenac and ketoprofen applied topically for acute or chronic benign sports-related soft-tissue injuries compared to a placebo demonstrated a significant therapeutic effectiveness of NSAIDs over the placebo. Including more than 10 000 subjects, this study was designed to evaluate the tolerance of the treatment. Its effectiveness criterion was a pain reduction of at least 50%. As stated above, NSAIDs were more effective than the placebo for acute injuries (maximum length of treatment: 1 week), with a relative benefit of 1.7 and a number needed to treat (NNT) of 3.9. For chronic injuries (maximum length of treatment: 2 weeks), the relative benefit was 2.0 and the NNT was 3.1 .
The recent development of NSAID patches allows a controlled extended release of the active substance over 12 to 24 hours. Several recent controlled studies, testing diclofenac patches and ketoprofen patches compared to a cream and/or a placebo, confirmed the effectiveness of patches. Again, the effectiveness criteria were the reduction of pain (50%) and the tolerance to the treatment on a visual analogic scale (VAS). However, using patches didn’t allow an accelerated return to sport .
1.3.2
Intramuscular injections
The only published study—of questionable quality—concerns ketorolac injections . This study concludes that there is no reduction of pain compared to ketorolac administered orally. However, the adverse effects, such as an increased global risk of hemorrhage and renal complications, seem to be worse. This form of administration, nonetheless, remains privileged in professional sports (i.e., “smart bombs”) for specific events .
1.4
Non-steroidal anti-inflammatory drugs and the musculoskeletal system
1.4.1
Ligament injuries
Acute ligament injuries are repaired in three phases:
- •
an initial inflammatory phase, including the cleaning of injured tissue;
- •
a proliferative phase, including the formation of collagen fibers;
- •
a remodeling phase, which can last several months, when controlled mechanical stress is applied to the injury.
The most available studies concern in-vitro or animal models and examine the use of piroxicam, ibuprofen and Cox-2 inhibitors for an injury to the knee’s medial collateral ligament . The results are contradictory: in the short term, joint function is sometimes improved; in the medium term, the ligament’s resistance to tension is increased, decreased or remains the same; in the long term, potentially deleterious effects on healing have been observed . In vitro, protein synthesis in human fibroblasts is also inhibited by indomethacin .
For humans, the most studied injuries are acute sprains of the ankle or knee. In one comparative study of a placebo versus ibuprofen, the authors concluded that pain and swelling was initially diminished, the joint amplitudes were improved, and the load-bearing capacity was restored more rapidly. Positive effects were observed up to the 7th day . However, other studies have shown that, after 6 months, groups treated with another NSAIDs (piroxicam 20 mg/day for 7 days) showed reduced joint amplitude, increased anterior laxity and a higher recidivism rate (25%) .
NSAIDs thus appear to have a positive impact on the initial evolution of an acute ligament injury, which argues for the administration of NSAIDs for 3 to 7 days . This could allow a more rapid return to the athletic activity. However, in the long term, this rapid return is likely to be to the detriment of good-quality healing.
1.4.2
Tendon injuries
The role of NSAIDs in the treatment of overload tendinopathy is uncertain and is subject to debate. In fact, chronic tendinopathy doesn’t present inflammatory reaction , other than in certain cases of bursitis or associated synovitis. Moreover, frequently, persistent neovascularization and neo-innervation could be partially responsible for the pain .
A meta-analysis identified 37 random clinical studies, of which 17 were placebo-controlled; this study highlighted that only short-term pain (7 to 10 days) is reduced, particularly in the shoulder. The analgesic effectiveness of NSAIDs is clearly less for elbows, patellar tendons and Achilles tendons. This meta-analysis didn’t mention any effect size calculated from the diverse studies included in the statistical analysis .
In the long term, there is no evidence that NSAIDs are effective for treating tendon injuries, whereas the risk of adverse effects rises. Above all, the healing of a tendinopathy does not seem to be modified by taking NSAIDs . Moreover, the analgesic effect of NSAIDs permits athletes to increase the stress on their tendons prematurely, and this stress compromises the long-term cure .
Of the acute attacks, short-term treatment (up to 14 days) with celecoxib or naproxen (compared to a placebo) appears to be potentially indicated for true shoulder bursitis and De Quervain’s tenosynovitis. The essential effectiveness criteria are a maximal reduction in pain intensity at rest and a good tolerance of the treatment.
1.4.3
Bone injuries
The Prostaglandin E (PGE) family plays an important role in bone homeostasis. The members of this family stimulate both bone resorption by increasing the number and the activity of osteoclasts and bone formation by increasing osteoblasts replication and differentiation . It is thus understood that any substance altering prostaglandin synthesis can have an impact on the bone. For example, the inhibiting effects of NSAIDs on bone formation can be used to prevent heterotopic ossification following prosthetic surgery . In the scientific literature, numerous animal studies have demonstrated a delay in bone consolidation when NSAIDs are taken, even the selective Cox-2 .
Human studies have produced equivocal results for acute bone trauma and after surgery. Most often, these studies are retrospective or reflect particular situations, such as vertebral fusion. The harmful effects of NSAIDs vary, according the substance chosen as well as how long the substance was administered. A delay in bone consolidation has been described many times . Because of these effects on bone formation, it is recommended to avoid NSAIDs at least during the first weeks after a fracture. Afterwards, even though certain harmful effects are absent, their use is not really justified; analgesics should be enough to take care of the pain. In cases of stress fractures, NSAIDs should not be used for the same reasons. Thus, we recommend avoiding the administration of NSAIDs, especially in the first week after a fracture and in cases of stress fractures.
1.4.4
Muscle injuries
The gravity of muscle injuries depends on the degree to which the muscle’s main constituents—the contractile tissue and the support tissue—are affected. Immediately following a rupture of the muscle fibers, myofiber necrosis and inflammation result, with the dominant presence of neutrophiles and macrophages. It is not yet known whether the role of these neutrophiles and macrophages is positive or negative . In fact, for some authors, the neutrophiles are responsible for liberating cytokines and free radicals, which could make the initial injury worse. In the near future, selective inhibitors of free radical production may be developed, which would be an innovative pharmacological treatment . In the repair process that follows the injury, first, the macrophages ingest the necrotic tissues in a process known as macrophage phagocytosis. These macrophages are also a source of cytokines and growth factors. Then, fibrous tissue is produced. A remodeling phase is then undertaken in order to regenerate new muscle fibers and to organize the fibrous tissue .
There are many animal models of muscular injuries treated with NSAIDs. However, since transposing these results to human beings may be risky and since the methodology of these studies varies, it is difficult to draw unequivocal conclusions. For this reason, we only report the results of studies conducted with humans.
For Delayed Onset Muscle Soreness (DOMS), taking an NSAID (ibuprofen; 1200 mg/day), versus taking an analgesic (acetaminophen; 4 g/day) or a placebo, didn’t modify the concentration of neutrophiles and macrophages in the muscles for the three groups in this study . Similarly, there was no difference between the three groups in terms of the secondary effectiveness criteria: the rate of creatine kinase (CK) or PGE2 and pain intensity. The same inhibition was observed for prostaglandins and protein synthesis, whether the NSAID or the analgesic was taken .
However, when an NSAID (indomethacin; 100 mg/day) was administered from 4 days prior to the DOMS to 4 days after the DOMS, a reduction of satellite cells induced by effort was observed ; these cells are essential for muscle regeneration and are dependant on the presence of prostaglandin. Thus, taking NSAIDs regularly, which happens frequently in some sports, may allow sufficient tissue concentrations to be reached to potentially modify the local response.
This hypothesis was also supported in a study reporting that, compared to a placebo, the prolonged administration of diclofenac, beginning 15 days before an unusual eccentric effort, allows the increase in CK to be limited and the period of discomfort linked to sore muscles to be decreased, which are the effectiveness criteria commonly used in studies about muscles soreness .
In the more traditional context of an acute muscle trauma (i.e., tears or strains), taking an NSAID (diclofenac; 150 mg/day) or an analgesic (meclofenamate; 300 mg/day) or a placebo didn’t modify the outcome for subjects with hamstring muscle injuries, who also benefited from physical therapy. The evolution of pain (primary effectiveness criterion) or swelling (measure of thigh circumference) or muscle strength (iso-kinetic evaluation) was not different in the three groups in this study until 7 days after the injury .
Finally, the use of NSAIDs is probably less debatable in cases of deep muscle contusions, a frequent source of Myositis ossificans. However, no prospective random controlled study has clearly documented the effectiveness of NSAIDs in preventing the appearance of heterotopic ossification. Nonetheless, a recent analysis in the literature recommends taking indomethacin or other NSAIDs for at least 7 days after an “at-risk” deep muscle contusion. This recommendation is based only on works documenting the positive effect of NSAIDs in preventing heterotopic ossification after prosthetic replacement, without mentioning an effect size calculated on the basis of all the referenced studies.
In summary, our review of the literature doesn’t allow us to draw conclusions about the formal interest of taking NSAIDs for acute muscle injuries, except perhaps to prevent apparition of DOMS after eccentric exercise or to prevent heterotopic ossification after deep muscles contusions. On the contrary, such action could be counter-productive, inhibiting prostaglandin formation as well as protein synthesis.
1.5
Conclusion
In sports medicine, especially for acute or chronic injuries of the musculoskeletal system, high-quality studies on the use of NSAIDs are still rare. Yet, using NSAIDs or analgesics to treat many sports injuries is a common practice among athletes. The systematic prescription of NSAIDs is certainly still too frequent. Their administration should respond to precise criteria. It seems obvious that sports medicine specialists should think about the fundamental problems involved.
In addition, NSAIDs are far from being without harmful consequences for musculoskeletal tissue repair and organic systems (e.g., digestive tract, kidneys). Can we justify masking the painful symptoms of athletes to allow them the shortest possible time away from their sport, perhaps at the detriment of their tissue healing in the medium term and their functional recuperation in the long term.
A reasonable use of these substances seems to be necessary. However, although the prescription of NSAIDs is justified in certain cases, it should always be the minimal effective dose and the shortest possible length of administration. Table 2 presents a summary of our conclusions based on our non-exhaustive review of the literature.








