Spontaneous Behavioral Recovery After Stroke in Humans
In the weeks following a stroke, human subjects generally display some degree of spontaneous behavioral recovery. Considerable variability exists, however (
1). Key principles identified to date are that most spontaneous recovery tends to occur within the first 3 months after stroke onset, cognitive deficits are more likely than motor deficits to show spontaneous gains beyond these 3 months (
2,
3,
4,
5); stroke survivors with more mild deficits achieve recovery quicker than those with more severe deficits (
6,
7,
8,
9,
10); and that different patterns of recovery can exist across different neurological domains within the same patient (
11,
12). Because of the differences in the rate and extent of recovery across neurological domains, restorative stroke trials might need to use behavioral outcome measures that focus on a single neurological domain rather than the global behavioral scales employed in acute stroke studies (
12).
Insights from Animal Studies
Studies in animal models of stroke have provided insight into the events underlying spontaneous behavioral recovery after stroke. These studies have particular value because animal studies are able to provide insight at the molecular and cellular levels.
A unilateral infarct is associated with a number of growth- and repair-related molecular events. In many cases, unilateral injury incites a bilateral response (
Table 81-1). These have been reviewed in detail elsewhere (
13,
14,
15,
16,
17,
18,
19). These include structural changes in axons, dendrites, and synapses; increased activation and migration of endogenous neural stems; plus changes in extracellular matrix, glia, and angiogenesis. The brain becomes excitable, for example, showing increased
N-methyl D-aspartate (NMDA) receptor binding and gamma-aminobutyric acid (GABA) receptor down-regulation (
20,
21). Cell cycle proteins and growth factors increase to levels far above the normal level for the adult mammalian brain (
22,
23,
24,
25). Many of these events are most pronounced in the peri-infarct area.
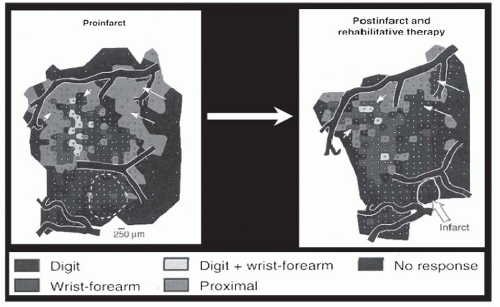
Insights have also come from studies of cortical representational maps. Nudo et al. (
26), in a landmark study, described changes in the hand motor map after stroke in subhuman primates. These authors found that hand motor training after stroke was associated with expansion of the motor cortex hand representation (
Fig. 81-1). This reorganization was later found to be related to local synaptogenesis (
27). Similar findings have been described in primary sensory cortex (
28). Other influences on cortical representational maps, such as demographics (
29,
30,
31), injury (
32,
33,
34,
35), medications (
36), experience (
17), and environment (
26,
37,
38,
39,
40), have also been described.
Magnetic resonance imaging (MRI) studies in animals have also provided insights into the basis of behavioral recovery from stroke. These are of particular value: they examine changes in representational maps noninvasively, and furthermore the metrics extracted can be very similar to the metrics reported in human studies; such direct comparisons might be very useful for effective translation of therapeutic advances. For example, changes in connectivity between sensorimotor cortex and deep gray matter structures in rats subjected to middle cerebral artery (MCA) stroke (
41) are similar to findings in human stroke patients (
42). Also, serial functional MRI (fMRI) studies examining rat upper limb have described a shift in laterality of activation after stroke such that early
after stroke, brain activation during affected paw stimulation is mainly in the contralesional sensorimotor cortex. Later after stroke, activity shifts toward the prestroke (normal) pattern in the ipsilesional sensorimotor cortex (
43,
44). These findings are closely aligned with findings from serial fMRI exams in human subjects with stroke (
45,
46,
47,
48,
49,
50).
Systems Insights from Brain Mapping Studies in Human Subjects
The molecular measurements from animal studies are difficult to obtain in humans, though occasionally specific questions can be probed with molecular imaging methods such as positron emission tomography (PET). Information regarding the brain events underlying stroke recovery in humans can be obtained, however, from studies of brain systems via brain mapping. A number of functional neuroimaging methods have been used to this extent.
Overall, after stroke in humans, tissue function is reduced within the injured (or for deep strokes, overlying/corresponding) primary neocortex (
51,
52,
53). Behaviors that rely on the dysfunctional primary cortex suffer. The best spontaneous return of behavior is associated with resolution of such reductions of cortical function. A number of compensatory brain responses also contribute to spontaneous behavioral recovery. These include increased activation in secondary areas that are normally connected to the injured zones through a distributed network, a shift in interhemispheric lateralization toward the contralesional hemisphere, and shifts in representational maps surrounding the infarcted zone. In many cases, the larger the injury or greater the deficits, the more these compensatory events are seen. These compensatory responses are tricks of the desperate, but in patients with injury-related deficits several lines of evidence suggest that often they are better present than absent. However, the best behavioral outcomes after stroke are associated with the greatest return of brain function toward the normal state of organization (
49,
53,
54,
55,
56,
57). These findings take on particular importance when it is realized that the same events that support
spontaneous recovery are likely those to be measured to assist studies aiming to therapeutically improve recovery (
58).
Stroke Injury Reduces Cortical Activity Locally
When a stroke injures primary cortex and/or its underlying white matter (and sometimes corresponding basal ganglia), cortical function is reduced initially, increasing over time (
45,
46,
47,
49,
56,
59). Thus, several lines of evidence suggest that final behavioral outcome at the end of the stroke recovery period is related to the degree of activity in primary cortex underlying the behavior under study (
60). For example, a study of patients with stroke who had reached a plateau in motor recovery found that the volume of primary sensorimotor cortex activation in the ipsilesional hemisphere during affected hand movement was related to the level of behavioral recovery (
53). Similar findings have also been described in language (
49,
55,
61,
62,
63) and right hemisphere attentional networks (
56). Consistent with this, transcranial magnetic stimulation (TMS) studies of motor cortex and its efferents after stroke have generally found that cortical motor maps are smaller and corticospinal tract physiological integrity is reduced in proportion to severity of clinical deficits (
64).
Viable cortical regions that surround an infarct might have special importance to repair and recovery. In animals, this peri-infarct zone often shows the greatest levels of growthrelated molecular changes after stroke (
14,
18,
26,
38,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76). Furthermore, amplification of peri-infarct repair-related events in animals by a therapeutic intervention has been associated with improved behavioral outcome (
69,
71,
77,
78). In humans, the volume of threatened but surviving peri-infarct tissue is directly related to final clinical outcome (
79,
80). Brain mapping studies in humans with chronic stroke have often noted peri-infarct activity (
55,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92), though the T2*-weighted MRI signal used to measure brain activation with fMRI is itself altered in the peri-infarct zone, perhaps due to glial scarring (
87). This observation complicates application of fMRI to the study of peri-infarct regions in patients with cortical stroke.
Stroke Injury Incites Increased Cortical Activity at Distant Sites
Three main forms of reorganization at sites distant from stroke have been described: (a) increased activity in brain regions distant from, but connected to, the stroke zone; (b) increased activity in the contralesional hemisphere, which reduces the extent to which interhemispheric balance is lateralized; and (c) somatotopic shifts within intact cortical regions. Each has been associated with behavioral improvement after stroke.
The first of these is increased activation within cortical areas that, prior to stroke, comprise a distributed network (
49,
63,
81,
83,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102). This has been described in many studies, indeed since the first poststroke functional imaging study by Brion et al. (
103). Reaction across a distributed network has been reported across many neurological domains including motor, language, attention, and visual functions.
A second form of reaction to stroke is reduced laterality of brain activity (
61,
81,
93,
94,
95,
96). Reduced laterality is a cardinal pattern of brain response to injury, having also been described in other neurological contexts such as epilepsy (
104), TBI (
105), primary progressive aphasia (
106), and MS (
107). Note that the degree of brain insult needed to incite reduced laterality might be much lower than previously appreciated (
108,
109).
The exact function served by increased contralesional activity after stroke remains to be clarified. To some extent, this can be seen as simply a contralesional example of increased brain activation at sites distant from stroke. Another interpretation is that this is a passive event, reflecting a reduced interhemispheric inhibition resulting from the stroke (
110,
111,
112,
113,
114,
115,
116). Changes in interhemispheric inhibition are the focus of much discussion (
114), and possibly of therapeutic value (
117,
118,
119), though the significance of such findings might differ across patient subgroups (
120). Another hypothesis is that the contralesional hemisphere assumes functions that were previously based in the ipsilesional hemisphere. However, the data do not support this, at least not directly and completely. For example, stimulating contralesional motor cortex with TMS does not result in movement of the affected hand (
121), and indeed might indicate greater pathology if such a response is seen (
122,
123). Contralesional hemisphere functions might be more substantial when stroke is prenatal (
124).
A third compensatory response to focal injury is reorganization of somatotopic maps. Such maps are normally present in multiple primary cortices including motor, sensory, auditory, and visual cortex, and such somatotopic organization also exists in white matter, basal ganglia, secondary neocortex, and the hemisphere ipsilateral to movement (
125,
126,
127,
128,
129,
130). Nonhuman primate studies have provided rich characterization of cortical map changes after ischemic injury, providing insight into the effects of rehabilitation as well as infarct size, and also describing novel axonal projections that can arise in this context (
13,
26,
37,
38,
131,
132). This process has been less studied in humans after a stroke, where most, but not all (
83,
133,
134), investigations have focused on the motor system. In the motor system, the large scale features of motor cortex somatotopy that are normally present (
135) are preserved though occasional exceptions have been described (
136). A shift of the motor cortex hand representation after stroke in the dorsal (
137), ventral (
53,
94,
98,
136,
138), or posterior (
82,
136,
139,
140,
141,
142) direction has been reported. Sites assuming such a shift in brain activation can demonstrate increased cortical thickness (
134).
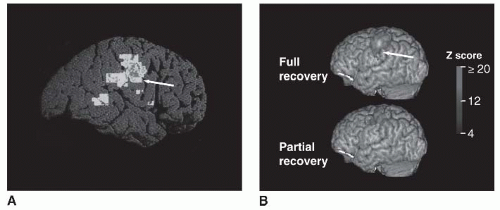
The motor cortex map relationships between face and hand may be among the most plastic in the brain, a suggestion that is supported by reports of invasion of hand motor representation into face motor area (
53,
98,
136,
138,
143,
144,
145) and face into hand (
146,
147), in a variety of settings, first described after stroke by Weiller et al. (
94). This process might reflect survival of distinct subsets of corticospinal tract fibers (
128,
148) that have key axonal connections between hand and face areas (
149), and so could theoretically represent an approach to identify a biologically distinct subgroup of stroke patients in whom a particular therapy (one that encourages a ventral hand map shift) might be most likely to succeed (
Fig. 81-2).
These forms of poststroke increased activation have certain principles in common. These changes are not epiphenomena,
as suggested by virtual lesion (
150,
151,
152,
153,
154) and other approaches (
155,
156,
157), but rather directly contribute to spontaneous behavioral recovery. Increased activity in distant areas (
46,
98,
158) and reduced laterality (
45,
48,
49,
54,
136,
159,
160) are time-dependent, increasing in the early weeks after stroke and generally declining thereafter. This decline is greater among subjects with better behavioral outcome, as the degree of persistently increased activity in both instances is generally highest in those with the poorest behavioral outcome (
54,
136,
161). However, note that persistent, increased reorganization is not always helpful, as persistence of poststroke plasticity can be associated with induction of epilepsy (
162) or chronic pain (
163).
Time Window Influences Application of Restorative Therapies after Stroke
The time window that defines a restorative therapy likely varies according to the therapy and biological target. The biological targets for restorative agents vary over time, with brain levels spontaneously rising then falling dramatically in the weeks following a stroke. Some models have broken down poststroke brain changes into three epochs (
160,
169,
170) that overlap at least in part: (a) acute injury, taking place in the initial hours after a stroke; (b) repair, starting in the first days after stroke onset and lasting several weeks. This is a golden period for initiating exogenous restorative therapies, as the greatest degree of spontaneous behavioral recovery is seen, and endogenous repair-related events (see
Table 81-1) reach peak levels (
Fig. 81-3). Note that exogenous therapies, from pharmacotherapies to behavioral interventions, must be examined in a two-tailed manner, as each can be deleterious
as well as potentially helpful, as discussed below; (c) a plateau, starting weeks-months after stroke, and representing a stable but still modifiable chronic phase. This third phase might have two temporal components (
98): one representing the beginning of the chronic phase, with associated loss of treatment windows from phase 2, and the other arising many monthsyears after stroke onset, associated with late changes and complications. Late changes and complications of a “late plateau” epoch include new dystonias, cognitive/affective changes, and spasticity/contracture.