Neural tube defects result from failure of the neural tube to close during embryogenesis. Although the incidence of neural tube defects has declined in recent decades, it remains the cause of chronic disability of between 70,000 and 100,000 individuals in the United States (
1). Myelomeningocele, also referred to as spina bifida, is the most common neural tube defect and is the most severely disabling birth defect compatible with survival (
1).
Myelomeningocele is a fluid filled cystic swelling, formed by dura and arachnoid. Myelodysplasia of the neural elements manifests in the vertebrae as a defect in the posterior elements. The sac protrudes through this defect and contains spinal nerve roots. Dysplasia of the spinal cord and nerve roots leads to bowel, bladder, motor, and sensory paralysis below the level of the lesion (
2). Patients with myelomeningocele can also have concomitant lesions of the spinal cord, such as diastematomyelia or hydromyelia, or structural abnormalities of the brain, such as hydrocephalus or Arnold-Chiari malformation, which can also compromise neurologic function.
The survival rate for patients with myelomeningocele in the 1950s was only about 10%. Due to advances in the management of several important complications, a recent series reported at least 75% of children born with an open myelomeningocele defect can be expected to reach their early adult years (
3). However, comprehensive treatment requires optimal care to prevent, monitor, and treat a variety of potential complications that can affect function, quality of life, and survival. This is best accomplished by a multidisciplinary team approach including specialists in orthopaedic surgery, neurosurgery, urology, rehabilitation, physical and occupational therapy, and orthotics. Access to nutritionists, social workers, wound specialists, and psychologists is also helpful.
As a result of the increased survival into adulthood, many patients with myelomeningocele now live long enough to eventually require transition to adult medical providers. This presents a great challenge since adult providers may lack the expertise necessary to manage these patients. Additionally, adults with myelomeningocele who failed to develop the skills they require to live independently remain dependent on aging family members who may be unable to care for them.
INCIDENCE
The incidence of infants born with neural tube defects shows regional and racial variations but is decreasing overall. The birth prevalence rate of myelomeningocele from 1983 to 1990 in the United States was 4.6 per 10,000 (
4). Since that time, there has been a decrease in the number of new cases of myelomeningocele. This decrease can be attributed to two main factors: prenatal screening with elective termination of affected pregnancies and increased awareness of the importance of administration of folate to women before and during pregnancy. The United States Public Health Service recommends that all women of childbearing age who are capable of becoming pregnant should consume 400 µg of folic acid per day for the purpose of reducing their risk of having a pregnancy affected with myelomeningocele or other neural tube defects (
5). Total folate consumption should be <1 mg per day because the effects of higher intakes are not well known (
5).
An estimated 50% to 70% of neural tube defects can be prevented through the daily consumption of 400 µg of folic acid (
5). The U.S. Food and Drug Administration mandated adding folic acid to all enriched cereal grain products by January 1998 (
6). From October 1998 to December 1999, the birth prevalence rate of myelomeningocele in the United States decreased 22.9% compared with 1995-1996 (
6). Notably, the prevalence of myelomeningocele remained higher among Hispanic women than among women in other racial/ethnic
populations (
6). This finding may be attributable to differences in folic acid consumption, eating habits, or genetic factors. In addition, religious and cultural preferences may play a role in the persistently higher prevalence of myelomeningocele in Hispanic women who may be less likely to terminate an affected pregnancy.
Overall the trend of decreasing incidence of myelomeningocele in the United States has continued after the folic acid mandate. From the early postfortification period of 1999-2000 to the recent postfortification period of 2003-2005, the birth prevalence of myelomeningocele among infants born to mothers of all racial/ethnic populations decreased 6.9%, from 2.04 to 1.90 cases per 10,000 live births (
6).
A similar trend of decreased incidence of myelomeningocele related to folic acid consumption has been reported in Europe. Two of the first European countries to develop a periconceptional folic acid supplementation policy were the United Kingdom (1992) and Ireland (1993) (
7). In a population-based study examining the effect of folic acid supplementation on the prevalence of neural tube defects in 16 European countries, a 32% decrease was found when comparing the periods 1989-1991 and 1999-2001 in the United Kingdom and Ireland (
7). A 17% reduction in prevalence of neural tube defects was found in countries with folic acid supplementation introduced by 1999. In contrast, a decrease of 9% was seen in countries with no supplementation policy by 1999.
ETIOLOGY
Myelomeningocele is believed to result from failure of fusion of the neural folds during neurulation, which occurs at 26 to 28 days of gestation. Conditions that result from abnormalities during the phase of closure of the neural tube, such as myelomeningocele and anencephaly, are referred to as neurulation defects. In contrast, conditions such as meningocele, lipomeningocele, and diastematomyelia arise from abnormalities that occur during the canalization phase from 28 to 48 days of gestation and are referred to as postneurulation defects.
The cause of this embryonic failure is not known but is suspected to be multifactorial in origin, involving both genetic and environmental factors. Folate deficiency is an important contributor to the cause of neural tube defects as evidenced by the decrease in incidence observed after folate supplementation. Other environmental factors have also been examined for a potential role in neural tube defects, including temperature; drug exposure; substance abuse; maternal infection; and other nutritional factors, such as vitamin B
12 and zinc (
8).
Genetic factors seem to play an important role in the development of myelomeningocele. Animal studies have shown as many as 100 mutant genes that affect neurulation, and almost all have homologs in humans (
8). Studies have suggested a higher incidence of neural tube defects in siblings of affected children than in the general population. A positive family history has been reported in 6% to 14% of cases (
9,
10). Overall, for a couple with a child with myelomeningocele, the chance that a subsequent sibling would be affected by a major malformation of the central nervous system is approximately 1 in 14 (
11). Although association with single gene defects, increased recurrence risk among siblings, and a higher frequency in twins than in singletons indicate a genetic contribution to the etiology, the low frequency of families with a significant number of neural tube defect cases makes research into genetic causation difficult (
8).
GENERAL HEALTH ISSUES
Patients with myelomeningocele have a high incidence of latex allergic reactions since they are exposed to latex products as a consequence of repeated surgical procedures, implantation of latex-containing materials, and catheterization (
23). Latex
allergy occurs in 18% to 40% of patients with myelomeningocele (
3,
23,
24). The reaction may be a severe, life-threatening anaphylaxis in up to 26% (
3). For this reason, it is imperative to avoid exposure to latex in myelomeningocele patients both in and out of the hospital environment. All surgical procedures performed on myelomeningocele patients should be done in a strictly latex-free setting.
Nutrition is an important issue in patients with myelomeningocele and appropriate counseling should begin at an early age. Childhood and adolescent obesity is common in patients with myelomeningocele and likely results from a variety of factors including energy intake and motor impairment. One study of 100 children and adolescents with myelomeningocele found 40% were markedly overweight, defined as Body Mass Index above the 95th percentile (
25).
The psychosocial impact of myelomeningocele on the patient and the family should not be overlooked. Parents of children with myelomeningocele report more psychosocial stresses compared to parents of able-bodied children. One study found parents of myelomeningocele patients reported less parental satisfaction, less perceived parental competence, more social isolation, and less adaptability to change in comparison to a matched group of parents of able-bodied children (
26). Another study looked at depressive symptoms in patients from 9 to 18 years of age with myelomeningocele compared to matched able-bodied patients. The authors found greater risk of depressive mood, low self-worth, and suicidal ideation in the myelomeningocele patients (
27). The multidisciplinary care team should be aware of the potential for psychosocial issues and be prepared to refer or treat any concerns appropriately.
PROGNOSIS FOR AMBULATION
Within the orthopaedic community, there is debate over whether or not working to achieve the goal of early ambulation in patients with myelomeningocele is worthwhile. Some attest early ambulation can provide physiologic and psychological benefits to a child with myelomeningocele even if that child will later become a sitter, while others dispute these benefits. One study compared a group of 36 high-level myelomeningocele patients who participated in a walking program with 36 matched patients who were prescribed a wheelchair early in life (
37). At final follow-up, only 12 patients in the walking group retained the ability for effective ambulation. Despite this, patients who had walked early had fewer fractures and pressure sores, were more independent, and were better able to transfer compared to the wheelchair group (
37).
Many factors influence the potential for ambulation in an individual patient with myelomeningocele. One of the most important is the motor level of involvement. Other contributing factors include sitting balance, upper extremity spasticity, obesity, age, and availability of appropriate orthotic support. Musculoskeletal deformity of the spine, pelvis, knees, and feet has also been shown to significantly influence ability to walk (
38).
Neurologic level of involvement and the resulting muscle group strength plays a crucial role in achieving and maintaining ambulation. Asher and Olson studied the ambulatory status of 98 patients with myelomeningocele and found a notable difference in the ability to walk between patients with fourth lumbar level of involvement and third lumbar level. Most of the patients with fourth lumbar level involvement were functional household or community ambulators compared to the third lumbar level patients, who were mostly nonfunctional ambulators (
38). In the same study, 20 of 21 patients with fifth lumbar or sacral level of involvement were community ambulators.
Maintenance of walking ability as an adult also correlates with the functional level of involvement. A review of 29 adult myelomeningocele patients aged 20 to 43 years found 95% of patients with third lumbar level of involvement or lower remained ambulatory (
39). In contrast, only 22% of patients with second lumbar level of involvement or higher remained ambulatory. The difficulty with maintaining the ability to walk as an adult relates to the high energy cost required to walk. Also, in patients with high level of involvement, there is a high incidence of spinal deformity requiring surgical treatment. Hip and knee flexion contractures are also common and prone to recurrence as an adult despite aggressive treatment during childhood (
17).
Correlating with functional level of involvement, one of the most important physical factors for maintaining ambulation is the strength of the quadriceps and the hamstrings muscles (
38,
40). Seitzberg et al. (
40) looked at a group of 32 patients with myelomeningocele and found a significantly better chance for maintaining ambulation as an adult if quadriceps strength was at least grade 4 during childhood. They also found that overall patients with grade 3 or higher hamstring function during childhood had a significantly better chance for adult ambulation. However, they noted that hamstring function was not relevant in patients with normal quadriceps strength (
40). Another study of 109 patients also found a correlation between quadriceps strength and ambulatory ability (
41). In this group, 82% of patients with grade 4 or higher quadriceps power were community ambulators, whereas 88% of patients with grade 2 or less were not functional ambulators.
The strength of the iliopsoas muscle has also been shown to be important for ambulation. McDonald et al. (
42) looked at a group of 291 patients with an average age of 14.5 years and found that 100% of the patients with symmetrical grade 4 or 5 iliopsoas strength were ambulatory. In contrast, 89% of the patients with iliopsoas strength grade 3 or less were nonambulatory.
Sitting balance is a factor that can be assessed at a young age and has also been shown to be predictive of ambulatory potential in patients with higher levels of involvement. The ability to sit without hand support indicates nearly normal functioning of the central nervous system. When hand support is needed for sitting, use of an orthosis and external support for ambulation is likely to be severely impaired (
17). A study of 206 patients with myelomeningocele confirmed that sitting balance was an independent predictor of community ambulation (
43). In this study, lumbar and sacral level patients with no sitting-balance deficit and sacral level patients with a mild sitting-balance deficit were likely to be independent ambulators.
FUNCTIONAL CLASSIFICATION
The best known and most widely used classification of myelomeningocele is based on the neurologic level of the lesion (
43,
44). Four main groups are identified based on the level of the lesion and associated functional and ambulatory capability (
Table 15-1).
Thoracic/High-Lumbar Level of Involvement.
The first group includes the thoracic and high-lumbar level patients, which represents approximately 30% of patients with myelomeningocele. This group is defined by the lack of functional quadriceps activity and has a neurologic level of L3 or above (
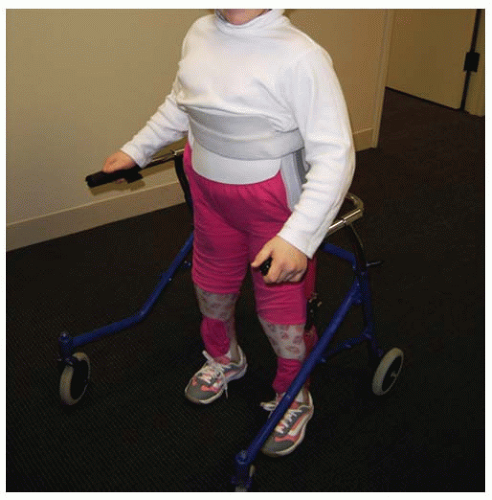
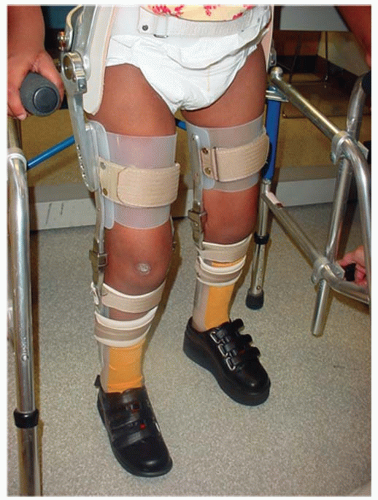
43). To achieve ambulation during childhood, patients in this group require bracing to the level of the pelvis with either a reciprocating gait orthosis (RGO) (
Fig. 15-1) or a hip-knee-ankle-foot orthosis (HKAFO) (
Fig. 15-2). The majority of patients in this group, between 70% and 99%, require a wheelchair for mobility as an adult (
17,
45). The inability to maintain community ambulation in adulthood relates to the high energy cost required to achieve ambulation with either an RGO or an HKAFO.
Low-Lumbar Level of Involvement.
The next group, approximately 30% of patients with myelomeningocele, has
low-lumbar level of involvement. Functionally patients in this group have purposeful (grade 3 or higher) quadriceps and medial hamstring activity but lack purposeful activity (below grade 2) of the gluteus medius, gluteus maximus, and gastrocsoleus muscles. Hence, these patients require braces to control the position of the foot and ankle as well as crutches or a walker in order to ambulate. Between 80% and 95% of patients in this group maintain community ambulation in adulthood, but
most will use a wheelchair for long-distance mobility (
43,
45). This group includes patients from L3 to L5 level of involvement, although patients with L3 level of involvement represent a transitional population and are included in this group only if they have evidence of strong quadriceps and medial hamstring function (
43). Since medial hamstring function is needed for community ambulation, there is a significant difference in the ability to walk between children with L3 and L4 level of involvement (
38). Because of this, children with L4 level of involvement have the most potential benefit from proper orthopaedic care of musculoskeletal deformities. Aggressive treatment of hip contractures; rotational malalignment of the tibia; and deformities of the knee, ankle, and foot are essential to maintain functional ambulation.
High-Sacral Level of Involvement.
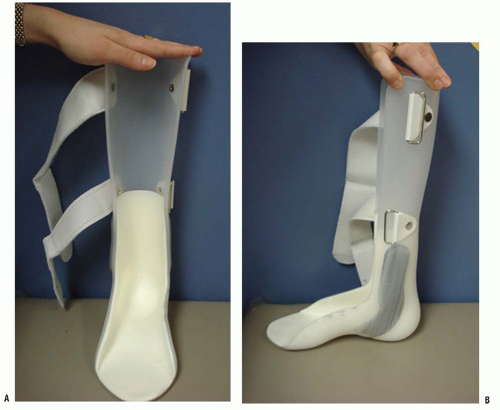
Patients with high-sacral level of involvement represent approximately 30% of patients with myelomeningocele. Patients in this group have functional activity in the quadriceps and gluteus medius (grade 2 or higher) but lack functional activity in the gastrocnemius-soleus. Patients with high-sacral level walk without assistive devices but do require an ankle-foot orthosis (AFO) (
Fig. 15-3). These children have a characteristic gluteus lurch with excessive pelvic obliquity and rotation during gait.
Low-Sacral Level of Involvement.
The last group of patients, approximately 5% to 10% of patients with myelomeningocele, has low-sacral level of involvement. These patients also have both quadriceps and gluteus medius function, but are distinguished from the high-sacral level patients based on the presence of gastrocnemius-soleus functional activity. Patients with low-sacral level of involvement walk without braces or assistive devices and have a gait pattern that is close to normal gait because they have normal gluteus medius and maximus function.
Between 94% and 100% of patients with sacral level involvement maintain community ambulation as adults (
38,
45,
46). In this group, aggressive treatment of tethered cord syndrome; avoidance of arthrodesis in the foot; and treatment of deformities of the knee, ankle, and foot are important to promote functional ambulation.
FUNCTIONAL MOBILITY SCALE
Many instruments specific to the pediatric population exist to assess quality of life, health status, physical function, and mobility in patients with physical disabilities. However, many of these instruments, such as the Pediatric Outcome Data Collection Instrument and the Child Health Questionnaire, are time consuming to administer and analyze. Because of this, the Functional Mobility Scale (FMS) was described in 2004 as a useful, simple tool to describe the more focused issue of functional mobility in children with disabilities and to aid communication between orthopaedic surgeons and health professionals (
47).
The FMS was initially devised to describe functional mobility in children with cerebral palsy, but the authors reported they had also successfully used it to assess children with myelomeningocele (
47). Recently, the FMS was used in a study by Battibugli et al. (
18) to compare function in groups of patients with myelomeningocele. The FMS is unique because it allows quick, practical scoring of mobility over three distinct distances representing mobility in the home (5 m), at school (50 m), and in the community (500 m). In this way, it is effective for distinguishing between groups of children with varying levels of disabilities and provides a means for standardized communication between health professionals (
47). The FMS has also been found to be sensitive to detect change after operative intervention (
47).
To apply the FMS, a child is given a score from one to six based on their walking ability for each of the three distances assessed (
Table 15-2). A score of one is used when a child uses a wheelchair, two for a walker, three for the use of two crutches, four for the use of one crutch or two walking sticks, five for a child who is independent on level surfaces, and six for a child who is independent on all surfaces. Two additional possible ratings are C for a child who crawls for mobility in the home and N for a child who does not complete the given distance. For example, a child who ambulates with crutches at home and at school and uses a wheelchair for long distances but would be an FMS 3,3,1.
Use of the FMS allows for an accurate clinical picture of a given patient’s functional status at a distinct point in time. Often parents or the patient may have difficulty choosing a single response to a question regarding function and will default to the highest level of function. This can impact interpretation of outcome studies if parents choose different responses at different time intervals when there has been little actual change in function (
47). A major advantage of the FMS is its ability to account separately for distances representing home, school, and the community hence addressing the complexities of functional mobility in the real world.
GAIT ANALYSIS
Gait analysis is defined as the systematic measurement, description, and assessment of quantities that characterize human locomotion (
48). Clinical gait analysis has received a great deal of attention in regard to its application to the treatment of children with cerebral palsy. Increasingly gait analysis is also being recognized as a valuable component of the comprehensive orthopaedic evaluation of patients with myelomeningocele. Its use has been reported in the literature for assessing various manifestations of myelomeningocele including hip subluxation/dislocation, lower extremity contracture, and rotational abnormalities (
49,
50,
51,
52 and
53). Two main groups of patients with myelomeningocele can especially benefit from gait analysis: (a) patients with a low-lumbar lesion who walk with external support and a below-knee orthosis and (b) patients with sacral-level lesions who walk with no external support and AFOs (
17). Studies have shown the average walking velocity for a patient with low lumbar-level involvement is 60% of normal (
50). The average walking velocity for a patient with high sacral-level involvement is approximately 70% of normal (
52).
The components of gait analysis may include kinematics, kinetics, electromyographic data, measurement of videotape recordings, energy expenditures, clinical observation, and foot pressure readings (
48). The data obtained from these areas are presented as graphic and numerical data (for kinematics, kinetics, and ground-reaction forces), as electromyographic activity, and as videotape recordings. All of these data are then analyzed by a clinician with training in the interpretation of gait studies and a report of the gait analysis is generated. Several high-quality, commercial gait analysis systems are now available. The more comprehensive of these systems provide the clinician with three-dimensional kinematics and kinetics as well as dynamic electromyography (
48). Three-dimensional gait analysis is especially useful for analyzing transverse plane deformities such as rotational problems. However, when a three-dimensional study is not available, the data obtained from a two-dimensional study have useful applications in the documentation of coronal and sagittal plane deformities such as crouch gait and foot deformities.
Kinematics describe the spatial movement of a body without consideration of the forces that cause the movement. These movements are linear and angular displacements, velocities, and accelerations. Kinematic data answer the question of what is happening at the level of each of the major lower extremity joints but not why it is happening (
54). Kinematics are useful
in determining treatment outcome through the comparison of preoperative and postoperative gait analysis data.
Kinetics on the other hand describe the mechanisms that cause movement around a joint. Hence, kinetics answer the question of why a particular movement or gait deviation occurs (
54). Kinetic data include ground-reaction forces, joint moments, and joint powers. In order to calculate kinetic data, simultaneous acquisition of joint motion and force-plate data is necessary (
48). The study of kinetics leads to improved understanding and knowledge of the pathogenesis of gait patterns (
54).
Gait analysis is useful in preoperative planning for ambulatory myelomeningocele patients because it allows accurate dynamic assessment of an individual patient’s gait problems. Postoperatively gait analysis is used to obtain a much more accurate, objective, and quantitative assessment of outcome than was previously possible (
54). Often a patient’s true functional status differs from what would be expected based on information obtained during the static clinical examination. Moen et al. demonstrated this in a study examining crouch gait in myelomeningocele patients. They found significantly greater dynamic knee flexion during ambulation using gait analysis than what was measured on clinical examination. Gait analysis is a useful component of the comprehensive evaluation of ambulatory myelomeningocele patients, especially when surgical treatment is being considered.
With specific regard to patients with myelomeningocele, gait analysis is useful to assess the abnormal movements that occur as compensation for muscle weakness. For example, due to weakness of the gluteus medius and maximus muscles, compensatory movements at the pelvis and hip such as increased active pelvic rotation and stance phase hip abduction develop to facilitate forward progression of the limb and maintain independent ambulation. All children with low lumbar-level involvement show increased anterior pelvic tilt, but compensatory movements become less pronounced with lower levels of motor involvement (
17).
Gait analysis is helpful in determining the course of treatment for patients with hip flexion-adduction contracture and low lumbar or sacral-level patients with unilateral hip subluxation or dislocation (
50). Gait analysis has also proved useful in increasing the appreciation of the effects of rotational malalignment of the lower extremity. Specifically, it has helped with understanding the relationship between external tibial torsion and a significantly increased valgus stress at the knee joint (
49). In addition, the information gained from gait analysis in regard to the coronal and transverse plane kinematics at the pelvis and hip and the coronal plane kinetics at the hip and knee is important in the prescription of effective orthotics and walking aids (
53).
OVERVIEW OF ORTHOPAEDIC CARE
Over the past 30 years, the overall care of children with myelomeningocele has changed substantially in regard to all specialties including neurosurgery, urology, rehabilitation, orthotics, and orthopaedics. Specifically relating to orthopaedics, the advent of gait analysis in the late 1980s contributed to a better understanding of the underlying deformities and their effect on function. This has led to a shift in the focus of orthopaedic treatment from the goal of radiographic changes to functional improvement (
55).
The main goal of orthopaedic care of a patient with myelomeningocele is to make the musculoskeletal system as functional as possible. As discussed earlier, walking ability is highly dependent on the neuromuscular level of the lesion. Whether or not ambulation should be the goal for every child with myelomeningocele is controversial. The role of the orthopaedic surgeon is to assist the patient and the family in developing realistic individualized goals and to provide the necessary care to meet these goals. Additionally, providers must help families to avoid neglecting the child’s total development while focusing on the use of the lower extremities. Emphasizing intellectual and personality development utilizing wheelchair mobility, wheelchair sports programs beginning in preschool, and educational mainstreaming can lead to dramatically increased independence (
17).
Both congenital and acquired orthopaedic deformities are seen in patients with myelomeningocele. Congenital deformities are present at birth and include kyphosis, hemivertebrae, teratologic hip dislocation, clubfoot, and vertical talus. Acquired developmental deformities are related to the level of involvement (
4) and are caused by paralysis, decreased sensation in the lower extremities, and muscle imbalance (
34). For example, calcaneus foot and hip dislocation are two acquired orthopaedic deformities caused in part by muscle imbalance. Orthopaedic deformities may also be result from iatrogenic injury such as postoperative tethered cord syndrome. Accordingly, the orthopaedic surgeon must monitor spinal balance and deformity and assist with monitoring the neurologic status of each patient.
The newborn examination of a patient with myelomeningocele should include identification of the level of paralysis for each extremity. Any associated conditions such as clubfoot or hip or knee contractures should be recognized and treated appropriately. In addition, a manual muscle test should be performed by a skilled physical therapist to evaluate the neurologic level of function. This should be done before closure of the spinal defect, again 10 to 14 days after closure, and then on an annual basis. Since a given patient’s motor level should remain the same throughout their lifetime, a change in muscle strength may be a sign of tethered spinal cord.
After the initial newborn examination, orthopaedic follow-up should occur regularly every 3 to 4 months during the 1st year of life. After that, patients are seen every 6 months until the age of 11 or 12 years after which time patients are followed annually. The follow-up periodic orthopaedic examination should include assessment and monitoring of motor and sensory function, spinal alignment, and skin integrity. Orthoses should be inspected on a regular basis to ensure appropriate fit with no areas of irritation or pressure points on the skin. Patients with myelomeningocele have multiple
medical comorbidities that must be considered as part of any orthopaedic treatment. Because of this, orthopaedic care should ideally be administered as part of a multidisciplinary team including neurosurgery, urology, and physiatry.