Chapter 43 Muscle Pain Syndromes
Muscle pain is common in clinical practice. The most frequently described muscle pain syndromes are myofascial pain syndrome (MPS) and fibromyalgia syndrome (FMS). Many question whether these two syndromes exist,13,158 but in the past 2 decades many well-controlled studies have strongly supported their existence. In light of this controversy, it is best to consider myofascial pain and fibromyalgia as clinical syndromes rather than distinct disease entities.
Etiology and Classification of Muscle Pain
Pathophysiologic Considerations in Muscle Pain
Muscle pain can be elicited by any irritation of the pain pathway from the muscle to the cerebral cortex. This can include peripheral sensitization of nociceptors in the muscle or central sensitization in the central nervous system (CNS).4,37,149
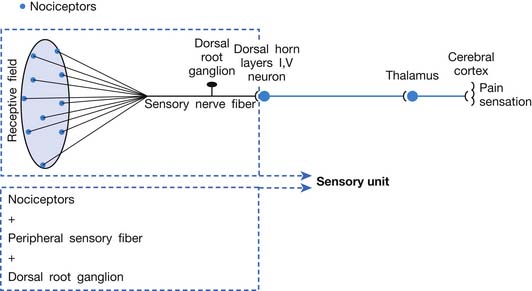
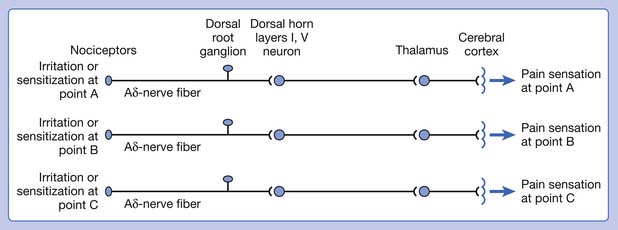
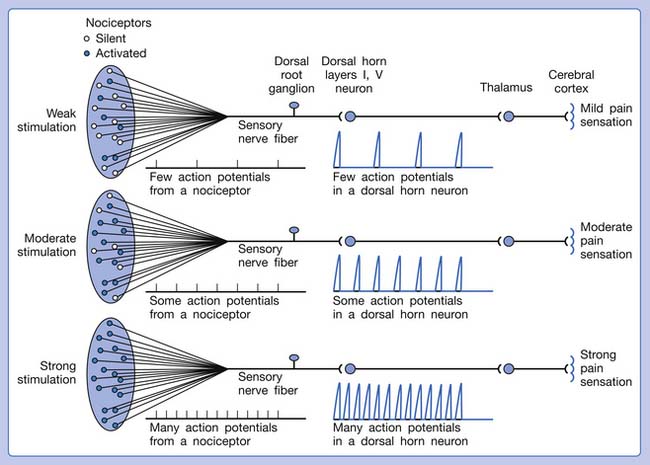
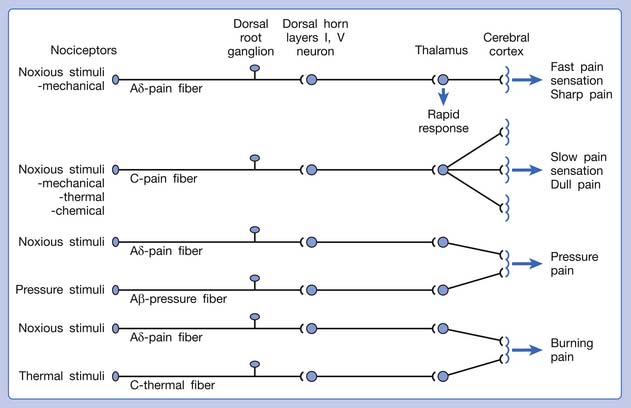
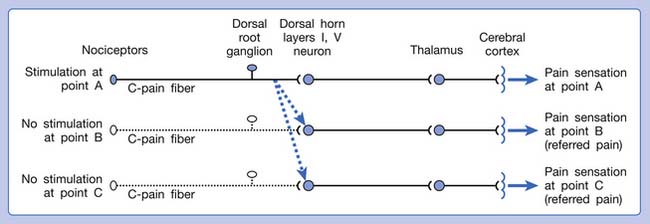
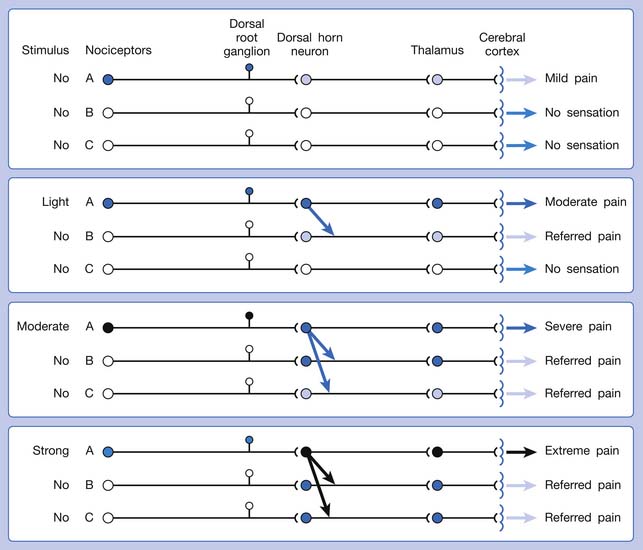
A basic peripheral “sensory unit” is defined as the dorsal root ganglion plus the peripheral nerve fibers (the peripheral axon and the centrally extended dendrite) and the supplied nociceptors (Figure 43-1). The location of pain is determined by the traditional physiologic concept of the law of projection. The cerebral cortex can perceive pain at a certain site via transmission of stimuli from the corresponding sensory unit to the dorsal horn of the spinal cord, and then via the spinothalamic tract to the thalamus and brain (Figure 43-2). The intensity of pain is determined by both the spatial and temporal summation of action potentials generated by the sensory unit (Figure 43-3). The discrimination of the character of pain is based on the simultaneous stimulation on two or more different sensory units with different entities (Figure 43-4).
The pain pathway for muscle pain appears to be different from that of skin. In fact, there is no evidence for the existence of an ascending tract that exclusively mediates muscle pain.149 After the first synapse with the dorsal horn cell in the spinal cord, the nociceptive information from muscle is largely mixed with information from other tissues. The dorsal horn cells receiving information from muscles are convergent neurons (both wide dynamic range neurons and nociceptive-specific neurons). The spinothalamic tract is the main ascending pathway for muscle pain in the spinal cord, but it also mediates pain from other tissues. A specific cortical “center” for muscle pain does not seem to exist.149 The cortical perception of the site, intensity, or characteristic of muscle pain is a complex process probably involving areas such as the anterior cingulate cortex, in addition to the primary sensory cortex.149
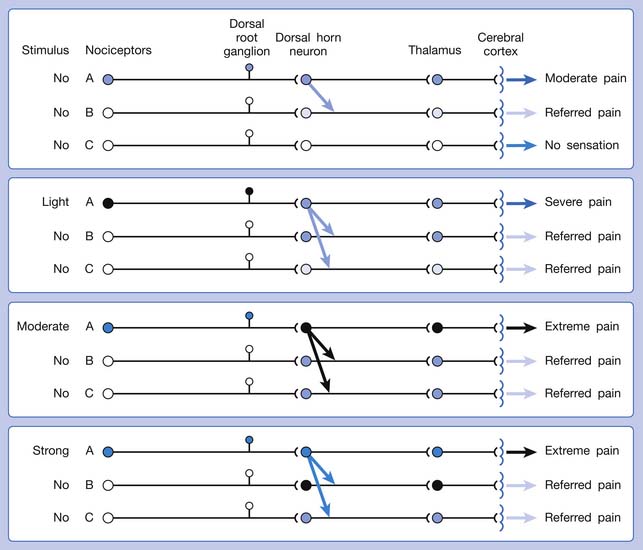
The ability of the CNS to react to a short-duration input of noxious stimulation with a long-lasting deviation from normal synaptic function (neuroplasticity) is particularly obvious in the case of muscle pain.4,149 Increased muscle sensitivity can be manifested as (1) increased pain intensity evoked by a noxious stimulus (hyperalgesia), (2) pain evoked by an innocuous stimulus (allodynia), or (3) increased size and number of referred pain areas with associated somatosensory changes.4
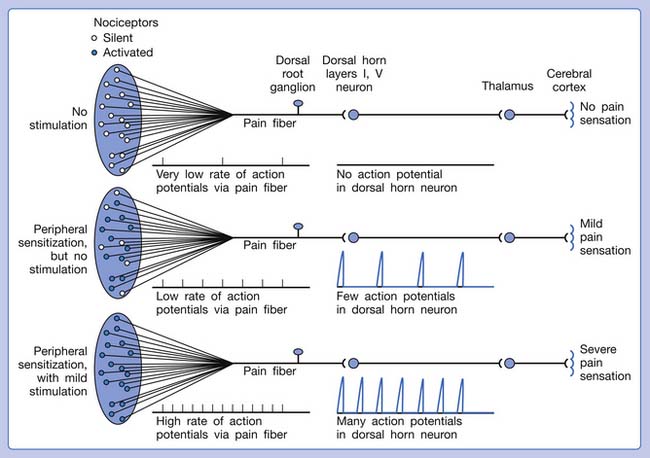
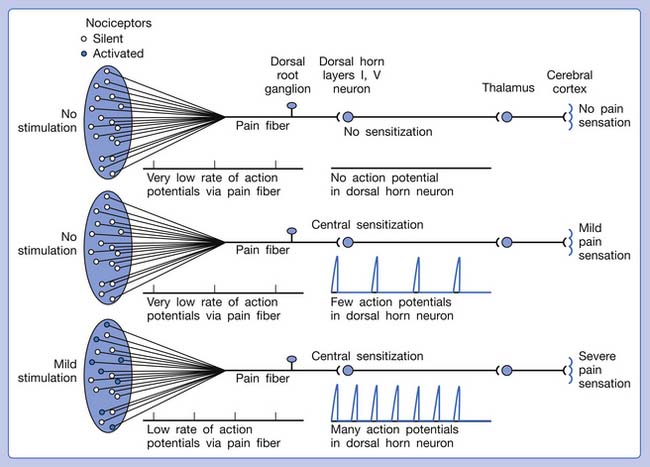
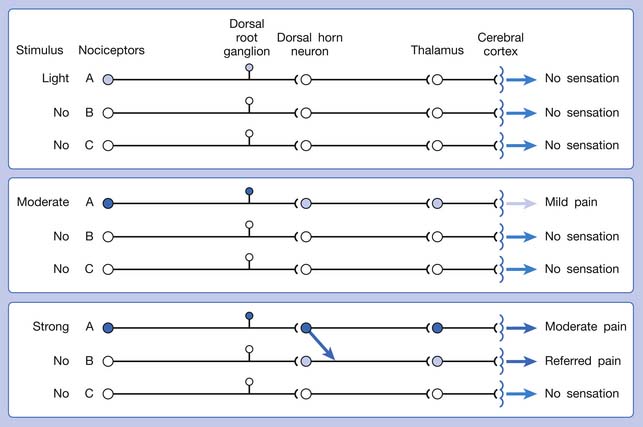
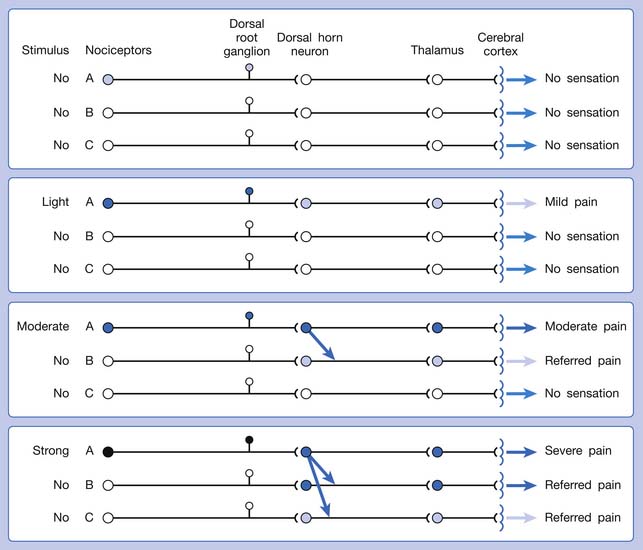
Referred pain can be elicited by a strong stimulation to a sensory unit with spread to the sensory pathways of different sensory units at the spinal cord (or higher) level of the CNS (Figure 43-5). Peripheral sensitization (nociceptor sensitization) occurs when there is an increase of excitability in the nociceptors of a sensory unit as a result of peripheral irritation (Figure 43-6). Central sensitization occurs when there is an increase of excitability of the spinal dorsal horn neuron (or higher center) for a long period.4,149 This is why a mild stimulus to a sensory unit can cause a cortical perception of severe pain in its receptive field (Figure 43-7).
Etiologic Classification of Muscle Pain
Based on its site along the pain pathway, muscle pain can be divided into five categories:
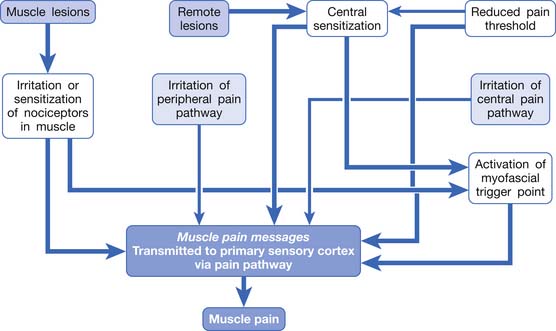
In summary, any irritation or sensitization of the muscle nociceptors or the muscle pain pathway can result in muscle pain (Figure 43-8). The causes of muscle pain are listed in Box 43-1. Russell has developed a system to classify soft tissue pain.162,163 In his system, focal muscle pain is localized pain, myofascial pain syndrome is regional pain, and fibromyalgia is generalized pain. Although this classification is convenient for clinical practice, it cannot provide the mechanism of muscle pain.
BOX 43-1 Etiologic Classification of Muscle Pain
Peripheral irritation or sensitization of the nociceptors in the muscle
Peripheral irritation (mechanical or chemical) to the peripheral nerve
Myofascial Pain Syndrome
Definition of Myofascial Pain Syndrome
Clinically MPS has been defined as a regional pain syndrome characterized by muscle pain caused by MTrPs.∗ In a broader sense, however, MPS includes a regional muscle pain syndrome of any soft tissue origin that is associated with muscle tenderness.53,149 In this chapter, MPS includes any pain phenomenon that is due to the activation of latent MTrPs. This can be secondary to pathologic conditions such as chronic repetitive minor muscle strain, poor posture, systemic diseases, and neuromusculoskeletal lesions (such as strain, sprain, enthesopathy, bursitis, arthritis, spinal disk lesion, etc.).87,98 Considerable evidence exists to suggest that myofascial pain syndrome is frequently caused by or related to a lesion in another soft tissue, as described in the next section.
Association of Myofascial Pain and Other Lesions (The Underlying Pathologic Lesions)
It can be observed clinically that although myofascial pain can be suppressed by an effective myofascial pain therapy, such as an MTrP injection, the pain often recurs a few days or weeks later if the related pathologic lesion is not eliminated.84,87,88 When the underlying etiologic lesion is completely eliminated, the MTrPs can be inactivated “permanently” unless reinjured. The underlying pathologic lesions are usually found in other regions remote to the activated MTrP. This remote activation of MTrP is due to central sensitization. Overuse or inappropriate use of a muscle can activate the MTrP in that muscle as a result of peripheral sensitization. Repetitive use of a muscle can reduce the pain threshold of the MTrP in that muscle.25 A recent study demonstrated that an MTrP (a sensitive spot in a palpable taut band with reduced pressure–pain threshold) of the extensor digitorum muscle can be induced by repeated eccentric exercise of that muscle.120 The MTrP can be successfully inactivated, however, by the avoidance of overuse or inappropriate use. Persistent or recurrent MTrPs are usually related to remote lesions, rather than to lesions in the affected muscle.
It has been reported that the number and pain intensity of MTrPs were significantly reduced after physical therapy or surgery for lumbar disk herniation.202 The association of active MTrPs with cervical disk lesions,111 cervical facet lesions,12 cervical radiculopathy,19 lumbar disk lesions,170 osteoarthritis of knee,6 teres minor tendinitis,199 lateral epicondylitis,47 floating kidney,109 septic arthritis,196 and herpes zoster26 has been demonstrated. Spinal adjustment107 and local injection132 of a cervical facet can effectively relieve the pain caused by MTrPs. It has been suggested that facet nociceptors and MTrP nociceptors might be connected in the spinal cord and might use the same nociceptive pathway to the higher center.12 Suppressing the facet pain suppresses the MTrP pain, and vice versa. However, during physical examination, compression of the related level facet joint can elicit pain in the MTrP regions, but needle stimulation to the associated MTrP can rarely induce pain in the correlated facet joint. Therefore facet dysfunction could be one of the important causes of the activation of MTrPs. It appears that, at least in some cases, MTrP pain is caused by the facet lesion or another musculoskeletal cause. It less frequently occurs as a consequence of primary muscle lesion.89,90,98 Activation of MTrPs can cause pain to avoid any movement that could interfere with the healing process of the primary lesion. Muscle pain can be an important defense mechanism to avoid further injury before complete healing of the etiologic lesion.89,98
Myofascial Pain Syndrome Controversies
The most important controversy in MPS is the problem of the lack of a set of specific diagnostic criteria. This set of agreed-on specific diagnostic criteria is required if different clinicians are to be able to agree on when it is present (with acceptable interrater reliability).152,158 Acceptable interrater reliability of many of the indicators of MTrPs appears to be obtainable only after the examiners have undergone special training.59
Although identification of an MTrP is clinically difficult, recent advances in MTrP research provide strong evidence that MTrPs exist. The electrophysiologic and morphologic findings of MTrPs cannot realistically be part of the criteria for the diagnosis of MTrP184 because they are expensive and time consuming. The practical diagnostic procedure still has to depend on a palpatory examination, which requires special clinical skills. Nonexpensive and simple devices for the measurement of MTrP characteristics are essential, and it is hoped that they can be developed in the near future.
Myofascial Trigger Point
Definition
Travell and Simons defined MTrP as the most tender (hyperirritable) circumscribed spot in a palpable taut band of skeletal muscle fibers.184,193 Pressure stimulation of a typical MTrP can elicit pain, referred pain, and LTR (brisk contraction of the muscle fibers in its taut band). The pain elicited by compression of this spot is familiar to the patient as the usual pain complaint (pain recognition).174
Latent MTrPs are tender but not spontaneously painful.184,193 They can be identified in most normal adult skeletal muscles but not in newborns or babies less than 1 year old.119 Active MTrPs are painful spontaneously or in response to movement of the involved muscle and are often so painful on palpation that the patient jumps (the “jump sign”). A latent MTrP can be observed clinically to evolve into an active MTrP. When an active MTrP is suppressed with treatment, it is still tender but not spontaneously painful because it has become a latent MTrP. Compression of the MTrP can reproduce or aggravate the patient’s usual complaint (pain recognition174), and elimination of the MTrP (or more appropriately, inactivation of the MTrP) can relieve the pain and other symptoms.
A myofascial pain patient can have many active MTrPs. Typically the syndrome begins with the patient having only one active MTrP in the affected muscle as a result of a soft tissue lesion. If it is not appropriately treated or the associated underlying pathologic lesion is not eliminated, the pain region can expand to other regions and develop additional active MTrPs. The original MTrP is called the primary MTrP or key MTrP, and the ones that occur later are called secondary MTrPs or satellite MTrPs.184 Inactivation of a key MTrP can subsequently eliminate the satellite MTrPs.84,184
Characteristics of Myofascial Trigger Points
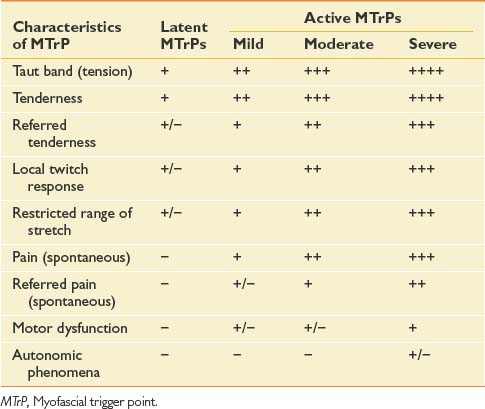
The chief characteristics of latent and active MTrPs are listed in Table 43-1. In clinical practice, active MTrPs can be further classified into categories based on the severity of pain and other characteristics. A clear-cut distinction exists between a latent MTrP and an active one, that being the existence of spontaneous pain. No clear distinctions, however, exist to categorize the severity of active MTrPs.
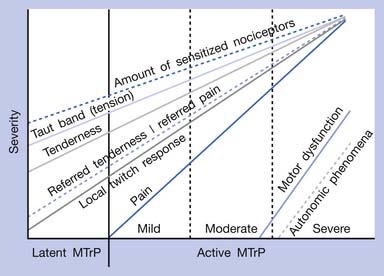
The separation of active MTrPs into three categories (mildly, moderately, and severely active MTrPs, listed in Table 43-1) is artificial. The common characteristics of any MTrP include taut band, spot tenderness, referred tenderness, LTR, and restricted range of stretch. Referred tenderness, LTR, and restricted range of stretch, however, are not always elicited in latent MTrPs. In a mildly active MTrP, it is painful, but the referred pain is often not obvious. In a moderately active MTrP, referred pain usually develops. Patients with severely active MTrPs typically show motor dysfunction and autonomic phenomena. As seen in Figure 43-9, for MTrPs the change in symptom severity is continuously variable. The degree of sensitized nociceptors in an MTrP region is the most important factor in determining the degree of MTrP severity.90 Any MTrP phenomenon (characteristic) shown in Figure 43-9 can be continuously variable and not necessarily follow the straight lines seen in the figure.
MTrPs are usually located within the end-plate zone because end-plate noise (EPN) can usually be recorded electromyographically in an MTrP region much more frequently than in other areas of normal muscle tissue.174,180,181,184 Simons defined this typical MTrP occurring in the end-plate zone as a central MTrP, and the trigger points in other locations in the muscle or tendon attachment region as attachment trigger points (A-TrP).184 The A-TrPs do not have all the characteristics of central MTrPs. A-TrPs are always located at the end of a taut band. Pain, referred pain, and LTR can be elicited, but EPN cannot be recorded from an A-TrP region of muscle.
Clinical Characteristics of Myofascial Trigger Points
Painful or Tender Spot
The most important characteristic of an MTrP is a circumscribed spot in the muscle with pain or tenderness. For most muscles, this spot can be identified in approximately the same region in different persons.193,194 The exact location of the MTrP in almost every skeletal muscle has been demonstrated in the “Trigger Point Manual.”184,193,194
Taut Band
Simons et al.174,184 have considered that an MTrP is always found in a taut band of skeletal muscle fibers, and a taut band is the precursor of an MTrP. A taut band is an essential component of the definition of an MTrP.174,184,193,194 Restriction of stretch with reduced range of motion can occur in a muscle with one or more tense taut bands that produced pain during stretch. This is different from muscle shortening caused by contraction because no muscle action potentials can be recorded from the muscle fibers in a taut band.149
Referred Tenderness and Referred Pain
Referred tenderness occurs when a distant muscle has pain in response to compression of an MTrP. Referred pain occurs when spontaneous pain is referred to remote sites from an MTrP.184 The occurrence of referred tenderness depends on two factors: the irritability of the MTrP and the pressure of compression.82,95,97 “Spontaneous” referred pain usually occurs clinically in relatively severe cases of MPS (see Figure 43-9).82 Each muscle tends to have the same area of referred pain in different persons.184,193,194 Figure 43-10 shows one example of the distribution of referred pain from an MTrP in the upper trapezius muscle. Regarding the location and referred pain pattern for all the exact muscles, please refer to the Travell and Simons texts.184,193,194
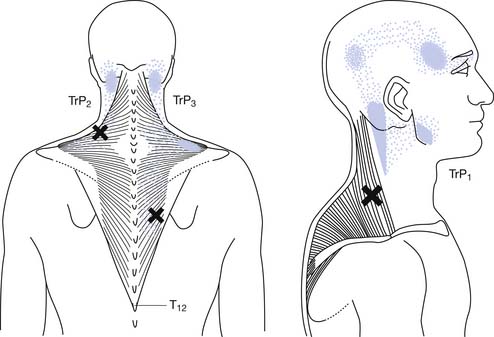
FIGURE 43-10 Referred pain pattern of myofascial trigger points in the upper trapezius muscle.
(Modified from Simons DG, Travell JG, Simons LS: trapezius muscle.In Travell & Simons’ myofascial pain and dysfunction: the trigger point manual, vol 1, ed 2, Baltimore, 1999, Williams & Wilkins, with permission.)
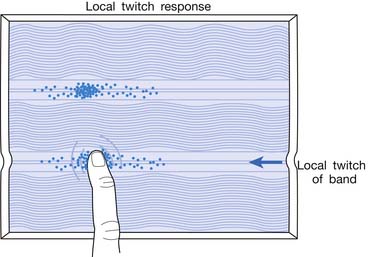
Local Twitch Responses
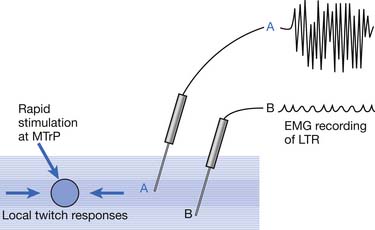
LTR is a sudden brisk contraction of a group of muscle fibers (usually in a taut band) in response to snapping palpation (quick compression across the muscle fibers perpendicularly of the MTrP) (Figure 43-11).184,193 The occurrence of the LTR also depends on the irritability of the MTrP and the pressure applied for eliciting LTR. High pressure is required to elicit an LTR in an MTrP with low irritability, and vice versa. A needle tip can provide high pressure stimulation to the MTrP and can elicit LTR much easier than using finger palpation.97,179
Motor Dysfunction
The clinically observed reduced muscle strength (weakness) caused by an MTrP is neither a true neurogenic nor a myogenic weakness. It is a pain-induced weakness and usually occurs only in severe cases of myofascial pain. Disuse muscle atrophy occurs rarely, mainly in cases of MPS with persistent severe pain.184 Other motor dysfunctions related to MTrP include increased responsiveness (muscle hyperactivity, referred muscle hyperactivity, referred inhibition), delayed relaxation, and increased fatigability (accelerated fatigability, delayed recovery).4,149 Mense and Simons149 called this hyperresponsiveness “muscle spasm” and “referred muscle spasm” and defined muscle spasm as involuntary contraction (with electromyographic [EMG] activity) of a muscle that is not dependent on posture.
Autonomic Phenomena
In extremely severe cases of MPS, autonomic phenomena (including abnormal sweating, abnormal tearing, abnormal salivation, increased vasomotor response, and increased pilomotor response) can be observed. For example, an MTrP in the sternocleidomastoideus muscle with high irritability can result in a discharge of tears and redness of the conjunctiva.193
The Basic Science of Myofascial Trigger Points
Myofascial Trigger Points Have Multiple Sensitive Loci
The concept of multiple sensitive loci in an MTrP region was first described by Hong88 and was based on clinical observations during MTrP injections. Using the traditional technique for MTrP injection with slow movement of the needle, pain and sometimes referred pain can be elicited when the needle tip encounters the tiny sensitive locus, but LTR can rarely be elicited by low-pressure stimulation (Figure 43-12). Hong84,86 has suggested a new injection technique by moving the needle in and out in a straight track and rapidly to avoid damage to the muscle fibers caused by side movement of the sharp-edged needle or resulting from the grabbing of the needle by an elicited LTR.184 An LTR can be easily elicited during rapid needle insertion (high-pressure stimulation because pressure = force/area, and force = mass × acceleration) when the needle tip encounters a sensitive locus (see Figure 43-12). Many LTRs can be elicited by needle insertions into different sensitive loci. More LTRs can be elicited in a painful MTrP region than in a region having low-grade pain. This is because the more sensitive loci (sensitized nociceptors) are located in the MTrP with high irritability rather than low irritability (see Figure 43-9). The sensitive locus has also been defined as an LTR locus because an LTR can be elicited by a strong pressure stimulation on this tiny locus.98 Histologic study revealed a nerve ending (nociceptor) at the LTR locus (Figure 43-13).93 Injection of certain dyes into the area of the nociceptors can cause the dye to spread up to the sensory neurons.128
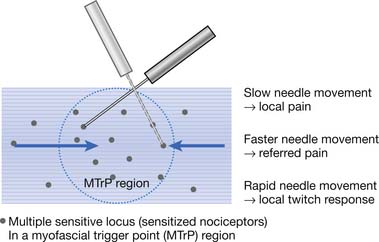
FIGURE 43-12 Pain, referred pain, and local twitch responses elicited during myofascial trigger point injection.
Animal Models for Myofascial Trigger Point Studies
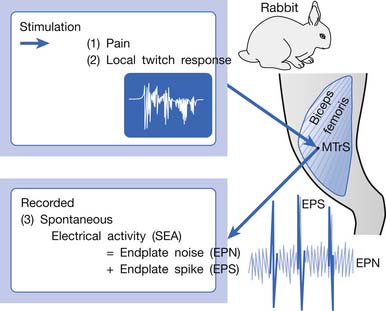
An animal model for myofascial pain study was initially reported in 1994.99 A sensitive spot could be found in the biceps femoris muscle of a rabbit. When this sensitive spot was compressed before anesthesia, the rabbit kicked and exhibited signs of pain. This spot was marked, and under anesthesia, a brisk muscle twitch that was similar to LTRs elicited in human skeletal muscle could be elicited by needle stimuli in the marked spot but rarely in other unmarked spots.99 Similar to human studies, spontaneous electrical activity (SEA), including EPN and end-plate spike (EPS), could be frequently recorded in this marked sensitive spot, but rarely from other sites in the end-plate zone and never in the non–end-plate zone.180,181 To distinguish it from human MTrP, this sensitive spot in the rabbit was defined as a myofascial trigger spot (MTrS).99 This animal model provided at least three important characteristics similar to human MTrP: pain, LTR, and SEA (Figure 43-14). Other similarities exist between human MTrP and rabbit MTrS as described below.98
Studies on Taut Band
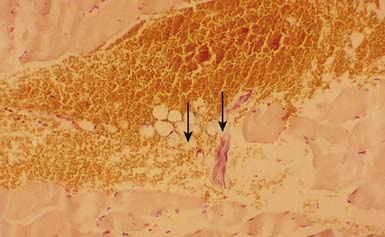
Electron microscopy has shown morphologic evidence of taut bands and contraction knots (or locally shortened sarcomeres) in the MTrP region (end-plate zone) of human muscles.157 In a light microscopic study with trichrome stain on canine muscles, Simons and Stolov182 demonstrated the contraction knot (Figure 43-15). A light microscopic study on rat muscles,150 an ultrasonic study on human muscles,58 and an magnetic resonance elastography study on human muscles24 have all shown the existence of taut band.
Studies on Myofascial Trigger Point Pain
To assess the effectiveness of a certain therapeutic modality or technique for myofascial pain relief, subjective pain intensity can be assessed with a numerical rating scale or a visual analogue scale (VAS). The scale usually ranges from 0 to 10, with 0 representing no pain and 10 the worst pain that could be experienced. The numerical rating scale is verbally reported by the patient based on the feeling of pain intensity. For VAS assessment, the patient is requested to mark the subjective feeling of pain at a 10-cm line on a card. The two ends of the line are marked with 0 and 10 to represent “no pain” and “worst pain,” respectively. The pain intensity is assessed by reading the distance between the 0 point and the marked point using the scale on the reverse side of the card.
Pressure algometer, developed by Andrew Fischer, is a useful tool to document MTrP irritability.49,51,82 The pressure applied on the MTrP can be read from a scale on this device as soon as the patient reports pain. Three consecutive measurements separated by intervals of at least a few minutes are required to confirm the consistency of repeated measures. This device can provide a semiobjective measurement because the patient cannot see the scale on the algometer. It has been reported that the pressure algometer is a reliable and valid device to help assess myofascial pain.154 The pressure algometer is also useful in assessing the effectiveness of MTrP therapy.∗
Spontaneous Electrical Activity Assessment
Recent studies have suggested that the irritability of an MTrP is proportional to the prevalence129 and the amplitude29 of EPN recorded from that MTrP region. It is likely that the measurement of SEA (including EPN) in an MTrP region can be used for the assessment of the effectiveness of a certain therapeutic method.†
Studies on Referred Pain
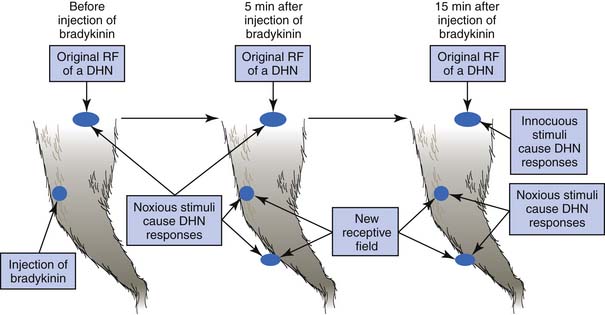
Referred pain from a muscle to another distant muscle has been demonstrated in animal studies.80,81,144–150 Pain signals from peripheral stimuli can be electrophysiologically recorded from sensory neurons in the dorsal horn of the spinal cord in a rat. The receptive field of a dorsal horn neuron can be identified by mapping the peripheral sites receiving stimuli. In a study by Hoheisel et al.81 (Figure 43-16) the original receptive field could be expanded to other sites 5 minutes after a noxious chemical substance was injected into another distant muscle. It appears that the brain can perceive pain at other sites in addition to the originally stimulated site. Fifteen minutes after a noxious injection, an innocuous stimulation could also induce a response in the original receptive field (allodynia). This phenomenon cannot be simply explained by the traditional “convergence–projection theory” (synaptic connections of a dorsal horn neuron with two separate innervation areas) because the size, number, and nature (high threshold or low threshold) of receptive fields for a dorsal horn neuron can be changed rapidly in the presence of noxious stimuli to muscle.80,112,149 Mense144,146,147 considered this mechanism to be secondary to the unmasking of formerly ineffective synaptic connections among neurons corresponding to different receptive fields. Many synaptic connections described in the convergence–projection theory are not functionally effective in a normal situation. However, some of these connections can become effective under the influence of certain conditions, such as a longstanding painful stimulus or a particularly strong painful stimulus.149 A strong noxious stimulus can send the impulse to the corresponding dorsal horn neuron and induce it to release substance P and calcitonin gene-related peptide (CGRP), which diffuse to other dorsal horn neurons and promote silent synaptic connections.148 This is a type of central sensitization.
In human studies, referred tenderness could also be elicited from a latent MTrP region or even normal muscle tissues if a strong pressure was applied.95,197 The pressure required to elicit referred pain from a compressed site is proportional to the degree of irritability (amount of sensitized nociceptors) at that site (Figures 43-17 to 43-21). The concept demonstrated in Figure 43-9 has been developed based on the above important studies.95,197 However, it is unclear why referred pain can also be elicited from a non-MTrP region, especially for that near an active MTrP. The non-MTrP region might also contain few nociceptors (see Figure 43-18). It is also likely that the high-pressure compression can indirectly stretch the muscle fibers in the taut band containing the MTrP.
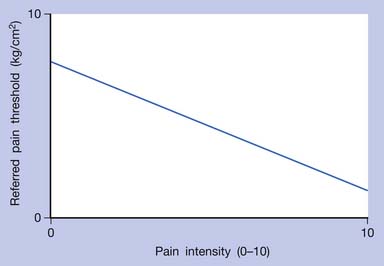
FIGURE 43-17 Referred pain threshold is proportionate to pain intensity of a myofascial trigger point.
Studies on the Local Twitch Response
EMG activity of an LTR elicited by stimulation of an MTrP can be recorded in the taut band containing that MTrP.54,179 LTR is most easily elicited when the MTrP, but not any other site, is mechanically stimulated (Figure 43-22) and is best recorded electromyographically in the taut band (Figure 43-23).99 In one case study, the LTR could not be electromyographically recorded from the extensor digitorum communis muscle in a patient with brachial plexus injury involving the posterior cord.91 The injured nerve partially recovered 6 months later, and then the LTR could be partially recorded.
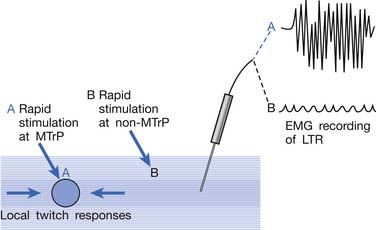
FIGURE 43-22 Local twitch responses (LTR) can be recorded only when the myofascial trigger point (MTrP) is stimulated.
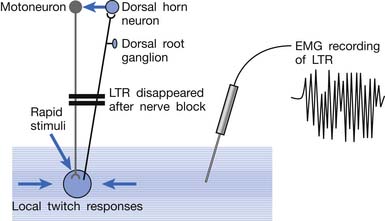
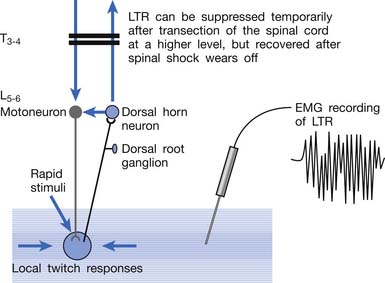
Animal studies have also demonstrated that LTRs can be elicited and electromyographically recorded from a muscle only if the innervated nerve is intact with a complete connection with the spinal cord (Figure 43-24).99 LTRs recorded from a muscle could be transiently suppressed after a complete transection of the spinal cord at a level higher than that providing innervation to that muscle but nearly completely recovered after the spinal shock period (Figure 43-25).100 It has been concluded that LTR is mediated via a spinal cord reflex.85,98,100 LTR can also be monitored by sonography.58
Surface Electromyographic Studies on Myofascial Trigger Point–Related Motor Dysfunction
As demonstrated in surface EMG, MTrP, as well as other soft tissue lesions such as ligamentous sprain or articular dysfunction, can induce muscle hyperactivity (so-called “muscle spasm”) in associated muscles.41,61,76,78,117 Because of muscle hyperactivity, the surface EMG amplitude recorded over a muscle with MTrP was 20% greater than the asymptomatic muscle. Headley76 further demonstrated that when pressure was applied on the MTrP of the supraspinatus muscle, a referred muscle hyperactivity could identified from surface EMG recordings in a distant muscle. Carlson et al.20 also found a significant reduction in surface EMG activity of the masseter muscle after MTrP injection of the trapezius muscle. On the other hand, referred inhibition of gluteal muscle hyperactivity by an active MTrP in the quadratus lumborum was demonstrated in surface EMG recordings.78 In studies on experimental acute muscle pain by Arendt-Nielsen’s group, reduced activation of the painful muscle was frequently observed.4,45,46,66,190 It is unclear why this finding is opposite to that seen in patients with chronic pain conditions.
Delayed relaxation of a muscle with active MTrP can occur during a repetitive exercise (alternative contraction and relaxation), and loss of relaxation at each end of contraction can be found.77,149
Acceleration fatigability with delayed recovery has been demonstrated in surface EMG studies.73,77,149 Evidence of initial fatigue (increased amplitude and reduced median power frequency of surface EMG activity) was found in a work tolerance study on the upper trapezius muscle with painful MTrPs compared with the contralateral pain-free muscle.73
Electrophysiologic Findings in a Myofascial Trigger Point Region: Spontaneous Electrical Activity (End-Plate Noise + End-Plate Spikes)
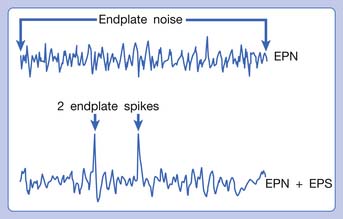
Hubbard and Berkoff114 were the first to record SEA from an MTrP region of the upper trapezius muscle in 1993. They suggested that SEA were potentials recorded from a muscle spindle.113 SEA can be recorded only in the end-plate zone, more frequently from an MTrP region than from other regions, including normal muscle tissues.∗ Further studies by Simons et al.180,181 indicated that there are two components in the SEA recorded from the MTrP region, including the low-grade continuous electrical activity and few sharp spikes with much higher amplitude (Figure 43-26). The low-grade continuous activity is the EPN, which is an accumulation of nonpropagated miniature end-plate potentials (MEPPs), and the spike is an EPS, which is a propagated action potential generated from the end-plate.174,176 EPS is probably elicited by a strong irritation of the recording needle on the “hyperirritable” end-plate because it occurs much frequently in hyperirritable active MTrPs than latent ones.129,174,176,180 When the SEA is recorded by an EMG needle, the patient always complains of a sharp pain sensation.180 This painful locus from where SEA is recorded was originally defined as an active locus,181 and later as an SEA locus98 or EPN locus.127 Simons174 has suggested that the occurrence of EPN indicates excessive leakage of acetylcholine (ACh) in the end-plate region based on extensive reviews of old literature. Previous studies indicated that only MEPPs were observed in a normal end-plate, and EPN were recorded only after a mechanical or chemical stimulation to the end-plate that induced excessive ACh leakage.116,138 These ACh molecules can cause calcium release from the t-tubules of the sarcomeres in the end-plate zone but not in other portions of muscle fibers. This is because EPN potentials are not propagated action potentials (no action potential can be recorded from a taut band). Muscle tension from a taut band is completely different from that caused by muscle hyperactivity (“spasm”) because propagated action potentials can be recorded from a muscle with hyperactivity secondary to a hyperirritable active MTrP.
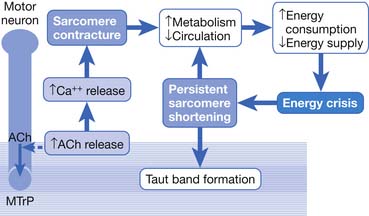
This focal contracture of sarcomeres can produce a contraction knot as shown in the electron microscopic findings (see Figure 43-15).182 Based on this finding, Simons183 brought an “energy crisis” hypothesis to explain the formation of a taut band (Figure 43-27). Sarcomere contracture can cause an increase in metabolism and a decrease in local circulation, so that the sarcomere cannot relax because of an inadequate energy supply. Sarcomere shortening occurs only in the end-plate region, but the sarcomeres outside the end-plate zone in either direction become somewhat elongated (because the muscle fiber length is unchanged) (see Figure 43-15). In this way, the muscle tension in the end-plate region is increased.
In an animal study, SEA persisted after transection of peripheral nerve and a high-level spinal cord.101 This results in the leakage of ACh in the end-plate region not being under the immediate control of the nervous system. In a single-fiber EMG study in rabbits, Kuan et al.130 found no increase in neuromuscular jitter in the MTrS region and suggested that the neuromuscular transmission itself was not impaired. This would indicate that the excessive ACh leakage is a secondary phenomenon and not caused by an abnormality in neuromuscular transmission. These findings support the theory that energy crisis in the MTrP region is a focal reaction and not related to neural controls. Once the nociceptors are involved in the integrated mechanism (see below), however, the spinal cord is involved in the mechanism of central phenomena such as pain, referred pain, and LTR. In a recent single-fiber EMG study, Chang et al.21 found evidence of degeneration in motor nerve endings in the MTrP region. Further study is required to clarify whether any motor nerve lesion is involved in the pathogenesis of MTrP.
Biochemicals Associated With Pain and Inflammation in a Myofascial Trigger Point Region
Using a microanalytic technique, Shah et al.171,172 measured biochemicals (associated with pain and inflammation) at GB-21 (acupuncture point in the MTrP region) in the upper trapezius muscle in subjects identified as active (having neck pain and MTrP), latent (no neck pain but with MTrP), or normal (no neck pain and no MTrP). They found that concentrations of all analyzed biochemicals were significantly higher in active than latent or normal subjects. It appears that substances related to either pain or inflammation are elevated in the vicinity of active MTrPs. These findings strongly support Simons’ integrated hypothesis of “energy crisis.”171,172 They also found a marked elevation of biochemicals during the LTR followed by a slow, variable return to baseline. Substance P and CGRP were the only two analytes for which concentrations during the recovery period after the LTR were significantly below the baseline concentrations.172 This interesting finding could possibly explain the immediate relief of pain (reduced substance P and CGRP) after eliciting LTRs during MTrP injection, but the overall mechanism is still unclear.
Studies on Myofascial Trigger Points in Early Life and Formation of Myofascial Trigger Points
Kao172 was the first to report that no latent MTrPs could be identified in children less than 1 year of age. It appears that latent MTrPs develop as the child is growing up. It is still unclear, however, when and how the nociceptors in the MTrP region become sensitized in later life. Gunn68,69 suggested that the formation of MTrP is due to minor lesions in the peripheral nerve, especially in the nerve root. This hypothesis cannot be generalized for all kinds of MTrPs because MTrPs can be activated as a result of a lesion other than nerve.83,98 It is not unlikely, however, that the formation of a latent MTrP in early life is related to minor peripheral nerve injury during the growing-up period.83 In clinical observation, the author has found that many adult patients with diffuse MTrPs in the upper back and shoulders related to cervical facet lesions have related neck injuries in early life.
Summary of Myofascial Pain Theory
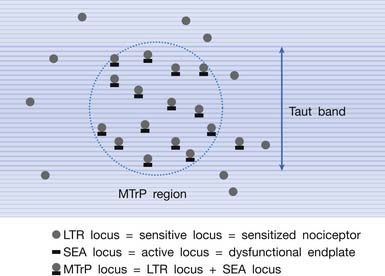
Multiple Myofascial Trigger Point Loci in the Myofascial Trigger Point Region
It has been hypothesized that there are multiple MTrP loci in an MTrP region (Figure 43-28).98 An MTrP locus contains a sensory component (the sensitive locus or LTR locus) and a motor component (the active locus or SEA locus). An LTR locus is a sensitized nociceptor (free nerve ending),93 and an SEA locus is a dysfunctional end-plate.174,176 An SEA locus is in the close vicinity of an LTR locus. They interact mutually for the formation of a taut band. Stimulation of an LTR locus can elicit pain, referred pain (as a result of central sensitization), and LTR (via spinal cord reflex).
Simons’ Integrated Hypothesis of Myofascial Trigger Point
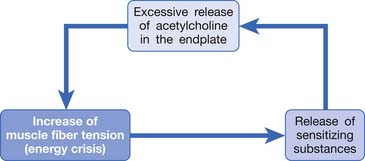
Based on the studies mentioned above, Simons et al.149,184 has postulated three essential features of MTrPs: excessive ACh release, sarcomere shortening, and release of sensitizing substances. These three essential features relate to one another in a positive feedback cycle (Figure 43-29) that is self-perpetuating once it is started (but which can be interrupted at several points in the cycle in a number of ways).149,177,178,184 An increased ACh release in the neuromuscular junctions (motor end-plates) can cause an increase of the muscle fiber tension. This results in a taut band that contains an MTrP and subsequently can cause “energy crisis” with increased metabolism and local ischemia with hypoxia, which can then induce secretion of sensitizing substances to cause pain. The sensitizing substances can then cause abnormal ACh release so that a vicious cycle is activated. It is unclear, however, whether the abnormal ACh release initially occurs to trigger the sensitization of nociceptors (peripheral sensitization) or the inflammatory reaction initially causes the release of inflammatory and pain substance171,172 and then induces abnormal ACh release. This is a “chicken–egg” question. In fact, Shah’s findings171,172 can support either one because the inflammation reaction can be secondary to the ischemic reaction in the contracture knot.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree