Ajit B. Pai
Isaac Darko
William Robbins
![]()
14: Moderate and Severe Traumatic Brain Injury
![]()
PATIENT CARE
GOALS
Provide patient care that is compassionate, appropriate, and effective for the treatment of moderate to severe traumatic brain injury (TBI) and the promotion of health.
OBJECTIVES
1. Identify appropriate components of a history and physical examination for a patient with TBI.
2. Discuss impairments and complications after TBI.
3. Describe a sample rehabilitation program for a patient with TBI.
Appropriate care for the patient with TBI includes many components. In the following paragraphs, we will discuss specifics related to the assessment and treatment of the adult with moderate to severe TBI. The medical history of a patient with TBI is highly important. Much can be gleaned from details at the time of injury, triage in the emergency department, surgical interventions, and so on.
HISTORY
History of Present Illness
Understanding the mechanism of injury (e.g., fall, motor vehicle accident, sport-related, assault, and blast-related) will allow providers insight into potential complications and allow for teams to educate persons with TBI and their families about future preventative measures. Traditionally, TBI is divided into primary and secondary injuries. Primary injury is the direct result of the force being applied to the head. It can be subdivided into open or closed injuries. Open head injuries result in the brain exposed to the outside environment due to a tear in the dura mater. This results in a higher risk of infection. Certain types of open head injuries result in more focal damage to the brain, such as blunt trauma and gunshot wounds. In closed head injuries the skull remains intact, resulting in mechanical and inertial forces that shear axons and lead to diffuse axonal injury (DAI) and contact forces associated with intracranial bleeds. Secondary injuries develop over the hours and days after the initial impact and are caused by bleeding, swelling, ischemic changes, complex biochemical changes, and changes in blood supply to the brain. Concomitant injuries such as musculoskeletal injuries and internal injuries (commonly seen in high-speed crashes and active combat injuries) should also be documented. In civilians, associated injuries are linked with more disability even 1 year after the injury (1).
Blast-related injuries can be subdivided into primary, secondary, tertiary, and quaternary. Primary injury represents the transduction of blast waves that damage tissue. These waves tend to also damage the hollow organs of the body. Secondary injury results from shrapnel and other objects traveling at high speeds due to the explosion. Tertiary injury is a result of the person being thrown from the blast into a stationary object. Finally, quaternary injury is due to hypoxic or toxic damage secondary to thermal or inhalation injuries.
TABLE 14.1 Severity Grading of TBI
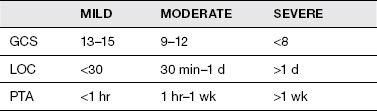
The severity of the brain injury should always be documented. Severity of injury (Table 14.1) is based on several measures: Glasgow Coma Scale (GCS) at time of injury, length of coma (LOC), and length of posttraumatic amnesia (PTA). If a person exhibits different grading of TBI based on the 3 measures, the severity is graded as the most severe (worst) of the measures. Mild TBI is defined as a GCS of 13 to 15; the patient is awake, may be confused, but is able to communicate and follow commands, and PTA is less than 1 hour. Moderate TBI is defined as a GCS of 9 to 12, an LOC between 30 minutes and 24 hours, and PTA less than 1 week. Severe TBI is defined as GCS of 8 or less, LOC greater than 24 hours, and PTA greater than 1 week. Intoxication at the time of the accident can affect GCS, as well as sedative medications given in the field.
Review of Systems
Symptoms such as headaches, weakness, dizziness, tinnitus, change in taste or smell, dysphagia, dysarthria, aphonia, vertigo, visual field deficits, decreased or altered sensation, focal weakness, and impaired balance can direct the provider’s attention to specific areas in the brain that may be injured. Understanding the symptoms allows a physiatrist to plan a treatment course.
Past Medical History
Previous TBI or stroke; previous pulmonary function and history of COPD or other lung disease; and previous psychiatric history including depression, anxiety, and personality disorders are important to understand in the treatment of persons with TBI.
Allergies and Medications
A complete list of allergies and medications is important in the care of patients with TBI. Medication reconciliation will allow the physiatrist to understand the medication of benefit and those that may be counterproductive to cognitive recovery.
Social History
Level of education and previous cognitive function, vocation and hobby history, socioeconomic status, home environment, and level of support from family should be documented and taken into consideration. The same is true for smoking, alcohol, and drug abuse history.
Functional History
Current and premorbid functional status is highly important to treatment planning. Recording both areas will allow a team to understand the baseline line of the patient and the rehabilitation potential.
PHYSICAL EXAMINATION
Cognition
Emergence from a disorder of consciousness is important early on in the treatment of TBI. Several scales are validated to monitor level of cognition in persons with disorders of consciousness, including the Coma–Near Coma and JFK Coma Recovery Scale Revised. Tests for awareness of self and environment, ability and consistency at which patient can follow simple and multistep commands, attention, concentration, memory testing of declarative and procedural memory, testing of PTA, and testing of higher cognitive function such as abstract thinking and judgment are important in the understanding of persons with cognitive disorders. Tests to help determine these areas include the Galveston Orientation and Amnesia Test, the Orientation Log, the Montreal Cognitive Assessment, and so on. The Rancho Los Amigos Scale allows standardized communication between rehabilitation team members about the person with TBI; it accounts for behavior and cognitive function.
Cognitive impairments are very common. Arousal is one of the most basic cognitive functions; it is fundamental in performing other higher levels of function and is often impaired following injury (2). Attention is commonly affected in patients with more severe TBI. Impaired attention can result in reduced information processing, impaired memory, and poor performance of multitask commands (3). Memory can be affected by many factors, but specific memory functions such as retrieval and consolidation are related to specific brain locations. Higher cognitive functions such as abstract thinking and judgment are often impaired.
Behavior
Behavioral changes are common to patients with moderate to severe TBI. These include mood disorders such as depression, anxiety disorders, and irritability. Screening for mood disorders such as depression and anxiety disorders will help in the rehabilitation of TBI. Depression after TBI can present as low mood, decreased interest, or anhedonia. Patients can complain of concentration and other cognitive issues, and certain behavioral changes might be seen, such as hyperactivity and disinhibition (4). In addition, irritability, akithesia, and restlessness are common after TBI. These symptoms may need to be treated to allow progression through the rehabilitation spectrum. Depending on the location of injury, agitation, disinhibition, and impulsivity can occur following moderate to severe TBI. Each of these problems can reduce the patient’s ability to participate in a rehabilitation program.
Musculoskeletal
Range of motion (ROM), concomitant fractures or dislocation, hemiplegia, hemiparesis, and increased tone are common after TBI. Spasticity, velocity-dependent increase in tone, is commonly graded using the Modified Ashworth Scale (Table 14.2). Gait is often altered after TBI, but the patient may be too functionally impaired to test.
TABLE 14.2 Modified Ashworth Scale
No increase in muscle tone | 0 |
Slight increase in muscle tone; catch and release or minimal resistance at the end of ROM | 1 |
Slight increase in muscle tone; catch followed by minimal resistance through <50% of ROM | 1+ |
Marked increase in muscle tone; resistance through >50% of ROM | 2 |
Considerable increase in muscle tone; passive ROM is difficult | 3 |
Increased muscle tone such that affected part is rigid in flexion or extension | 4 |
Concomitant musculoskeletal injuries often limit ROM. Limited ROM can affect mobility and ambulation, resulting in poor functional outcome. Upper motor neuron damage resulting in increased tone and spasticity is associated with more severe injury, spinal cord injury, anoxic injury, and older age.
Neurologic
Focal neurologic injuries are associated with the location and severity of the injury. Cranial nerve injuries resulting in facial weakness, dysphagia, dysarthria, and visual dysfunction are common, as well as vestibular dysfunction. Cranial nerve testing for nystagmus, tracking, visual field defects, blurred vision, facial weakness, facial sensation, gag reflex, hearing, taste, and smell will help determine focal nerve injury. The sense of taste and smell is often altered after TBI. Sensation to light touch, sharp touch, and proprioception can do the same. Check for increased deep tendon reflexes, as well as other tests for upper motor neuron (UMN) lesions such as Hoffman and Babinski reflex. Patients with more severe injury might show primitive and/or brainstem reflex. Romberg test, fine motor testing, and rapid alternating movements are useful in determining cerebellar involvement. Hemiplegia and hemiparesis in specific distribution can be related to the location of the brain injury. Diffuse weakness can be a result of deconditioning related to prolonged hospitalization. Autonomic dysfunction can also occur after brain injury.
General Examination
Skin breakdown and pressure ulcers are common after prolonged hospitalization. Providers should check the occiput, shoulders, sacrum, greater trochanters, elbows, and heels. Also check for skin integrity around the tracheostomy site and gastrostomy tube. Respiratory examination should include checking for amount and quality of sputum production. Abdominal distension and constipation are both common as a result of lack of mobility. Bowel incontinence is associated with more severe injury. Diarrhea can be associated with enteral feeds and infections. Check for amount and quality of urine and indwelling catheter integrity.
IMPAIRMENTS, ACTIVITY LIMITATIONS, AND PARTICIPATION RESTRICTIONS
More severe TBIs are related to increased activity limitation and participation restrictions. Activity limitation is an inability to execute a certain class of movement or cognitive function (i.e., difficulty bathing or dressing). A participation restriction is an inability to complete a task that most others are able to complete secondary to an activity limitation (i.e., maintaining employment or returning to school).
COMPLICATIONS OF MODERATE AND SEVERE TBI
There are many potential complications that can occur after TBI. Table 14.3 provides a checklist.
Autonomic dysfunction, oftentimes referred to as dysautonomia or paroxysmal autonomic instability and dystonia (PAID), is characterized by hypertension, tachycardia, and diaphoresis. This can occur any time after TBI and is due to sympathetic storming.
TABLE 14.3 Common Problems After TBI
Autonomic dysfunction |
Constipation |
Heterotopic ossification |
Insomnia |
Normal pressure hydrocephalus |
Posttraumatic headaches |
Posttraumatic seizure |
Spasticity |
Venous thromboembolism (VTE) |
Constipation occurs often after TBI due to immobility. It is prevented with hydration and an aggressive bowel program with stimulants and softeners. Once a patient is on a stable bowel regimen, then a slow wean of bowel medications can start.
Heterotopic ossification (HO) occurs after TBI; it usually affects the hips, shoulders, and elbows. Preventative measures for HO include ROM exercises, nonsteroidal anti-inflammatory drug (NSAID) medication, radiation treatment, and calcium-binding chelating agents, although in TBI there is no supporting literature for any of these treatments.
After TBI, sleep disorders are common and can be managed medically and through environmental control. These problems include insomnia and obstructive sleep apnea. At times, a sleep study can assist in diagnosing issues related to sleep.
Hydrocephalus may or may not be associated with elevated pressure. It is best managed with early detection of clinical signs and symptoms (cognitive decline including altered mental status, urinary incontinence, gait instability, headaches, etc.) as well as findings on imaging or large-volume spinal tap, it is commonly treated with neurosurgical placement of a ventriculoperitoneal shunt, although shunts can drain into the heart, pleura, or pelvis.
Posttraumatic headaches develop within 1 week of trauma and are classified based on traditional headache classification. Posttraumatic headaches can be managed nonpharmacologically using relaxation, biofeedback, and avoidance of triggers. They can also be managed pharmacologically using abortive agents such as NSAIDs, acetaminophen, and serotonin receptor agonists. Prophylactic treatment includes beta-blockers, calcium channel blockers, antidepressants, and anticonvulsants. One must rule out increased intracranial pressure, vascular injury, cerebrospinal fluid (CSF) leak, infection, and other serious complications that can present as headache.
Posttraumatic seizures (PTSs) are classified as immediate, which occur within 1 day of injury; early. Occurring within 1 week of injury; and late, occurring after 1 week. Prophylactic phenytoin for 7 days following injury can prevent early seizure, but not late PTS or posttraumatic epilepsy. Many acute care providers will use other antiepileptics such as levetiracetam, carbamazepine, or valproic acid. Patients who have had an early or late PTS are at higher risk for future seizures and may require longer treatment.
Appropriate venous thromboembolism (VTE) prophylaxis, speech therapy, and enteral feeding methods can prevent other common injuries such as blood clots and aspiration. It is important for the physiatrist to remain vigilant for these complications in order to quickly diagnose and treat appropriately.
SAMPLE REHABILITATION PROGRAM
A typical inpatient rehabilitation program for patients with TBI will include physiatrist oversight for medical management; 24-hour nursing to assist with medication management and daily care needs; physical therapy for mobility and transfer deficits; occupational therapy for activity of daily living (ADL) retraining; speech therapy for cognition, communication, and swallowing deficits; recreational therapist for community reentry; psychology for adjustment; and social work for case management. In addition, other useful team members will include a low vision therapist as TBI often creates difficulties with vision; neuropsychologist for return to work or school treatment planning; dietician for optimal caloric intake; wound care specialist; pharmacist; and patient/family educator. Most programs offer 6 or 7 days a week of therapy, and most days should consist of more than 3 hours of therapy a day. The daily schedule should be structured, as this will allow patients with cognitive or behavioral problems to adjust more quickly and help prevent agitation or irritability. Priority should be placed on sleep hygiene, stimulation reduction, and pain control. Additionally, the rehabilitation program should include a robust education program that incorporates both patients and social support.
A typical outpatient brain injury rehabilitation program includes interdisciplinary interactions with a physiatrist, case manager, physical therapist, occupational therapist, speech and language pathologist, and psychologist. If an interdisciplinary setting is not available, then the physiatrist should coordinate care between all individuals in a multidisciplinary manner. In the outpatient setting, the person with TBI may occasionally work with a recreational therapist when engaging in new activities or a neuropsychologist for testing prior to starting new schoolwork or an occupation. In addition, the physiatrist should be available for consultation for the patient’s primary care physician.
MEDICAL KNOWLEDGE
GOAL
Demonstrate knowledge of established and evolving biomedical, clinical, epidemiological, and sociobehavioral sciences pertaining to the field of TBI, as well as the application of this knowledge to guide holistic patient care.
OBJECTIVES
1. Explain the anatomy, physiology, and pathophysiology of moderate to severe TBI.
2. Discuss treatment options of complications of moderate to severe TBI.
3. Discuss ethical issues involved in managing a person with moderate to severe TBI.
ANATOMY
The brain is protected by the scalp, skull, and the dura, which is made up of three layers: the dura mater, which lines the skull; the arachnoid mater, a film that covers the entire brain and contains blood vessels; and the pia mater, which contains blood vessels that reach deep into the brain. Between the arachnoid and the pia is the subarachnoid space, which contains the CSF. CSF is formed in the choroid plexus and circulates through the ventricles into the subarachnoid space and then returns to the dural veins by the arachnoid villi. The brain is a complicated structure containing many parts. The cerebrum is divided into four lobes that lie above the cerebellum. The base of the skull includes multiple bony ridges that abut the brain parenchyma of the anterior temporal and inferior frontal lobes. This predisposes those areas to injury during trauma.
PHYSIOLOGY
The brain is made up of neurons, which consist of the soma, or body; dendrites, which receive communication; the axon, which is a long slender tube that carries information away from the cell; and the terminal buttons, which branch off from the axon and secrete neurotransmitters. Each neuron synapses with another neuron within the brain, the spinal cord, or end organs (in the case of cranial nerves). Neurons are typically colocated with neurons of similar function. Pockets of neurons make nuclei.
PATHOPHYSIOLOGY
Following initial injury, the brain undergoes a complex metabolic, chemical, and neurochemical cascade. Cell death occurs as a result of both apoptosis and cellular necrosis. Secondary injury also results in cerebral edema, elevated intracranial pressure (ICP), and decreased cerebral perfusion pressure. Excitatory amino acids (EAAs) are released, resulting in an influx of sodium and an increase of intracellular calcium, resulting in cellular swelling causing delayed injury and cell death, respectively. Certain areas of the brain, like the hippocampus, have a higher distribution of EAA-sensitive receptors that contribute to injury. Elevated lactate levels are also seen post injury and are thought to be neuroprotective. Lactate is also important for metabolism in the injured state. Inflammation is characterized by cytokine release including interleukins and tumor necrosis factor which can lead to secondary injury via blood brain barrier disruption, cerebral edema, and cell death. At later time points, cytokines may support neuroprotection and neurorepair through their effects on neurotrophin production (2). Several neurotransmitters are affected after injury; dopaminergic and noradrenergic systems are disrupted. Cholinergic transmission in the hippocampus is also decreased.
BIOMECHANICS
Contact forces occur when the head is struck in a fixed position. Inertial forces occur when the head is set in motion and accelerates. Inertial forces associated with angular acceleration can cause DAI, which is a result of tensile strain causing disruption of the axons. Superficial axons and axons in the gray–white matter junction are most vulnerable. Neurons in the corpus collosum and midbrain also are susceptible to DAI. Inertial forces associated with translational acceleration result in more focal injuries, such as contusions. Contusions at the site of impact are termed coup injuries and contusions at the opposite side of impact resulting in the brain’s impact with the skull are termed contrecoup injuries. As described earlier, the frontal and anterior temporal lobes are most susceptible to contusion due to their location close to skull ridges. Epidural hematomas (Figure 14.1) result from local impact and injury to dural veins and arteries. Subdural hematomas (Figure 14.2) result from inertial forces and tearing of bridging veins and are associated with falls. Subarachnoid hemorrhage (Figure 14.3) results from angular acceleration and shearing of vessels located in the subarachnoid space.
EPIDEMIOLOGY
The two age groups most at risk are 0 to 4 years and 15 to 19 years. Motor vehicle accident is the leading cause of TBI in the 15 to 19 age group. Also, younger individuals have a tendency to engage in higher-risk behaviors. Persons over the age of 65 are at the highest risk of fall-related TBI. Men are at higher risk than women, and persons of lower socioeconomic status are also at high risk. Military personnel have a higher risk of TBI related to blast injury and other combat-related injuries. Each year there are over 1.7 million occurrences of TBI in the United States (5). Only 25% of those are moderate to severe injuries (6). Two studies show that the cost of TBI in the year 2000 was upward of $75 billion in the United States; 90% of the total costs of TBI are attributed to severe injuries (7,8).
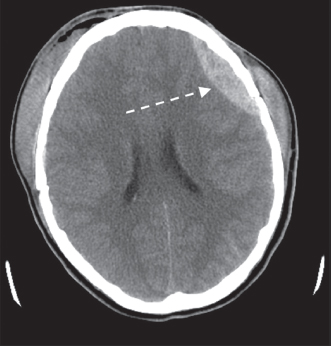
FIGURE 14.1 Epidural Hematoma
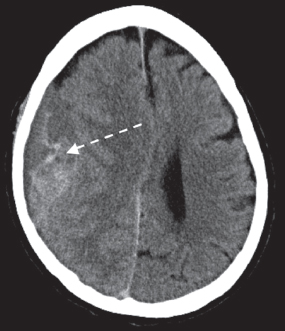
FIGURE 14.2 Subdural Hematoma
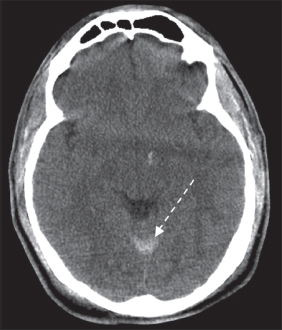
FIGURE 14.3 Subarachnoid Hematoma
TREATMENT
Although evidence for neuropharmacologic treatment is sparse, many providers choose to treat various conditions with medication. Cognitive impairment can be treated with amantadine in patients with a disorder of consciousness. Giacino et al. found that it accelerates the pace of functional recovery during active treatment (9). Although the mechanism of action is unclear, amantadine appears to act as an N-methyl-D-aspartate antagonist and indirect dopamine agonist. It also possesses noradrenergic properties that may affect a variety of cognitive domains. Additionally, it is thought to improve initiation. Side effects include a reduced seizure threshold, although, in Giacino’s study, there were no significant differences in adverse events as compared to placebo. Methylphenidate can increase dopamine and norepinephrine in the cortical and subcortical areas (10,11). It is commonly used to improve processing speed and attention as reported by multiple researchers. Side effects of methylphenidate include headache, insomnia, nausea, dizziness, and anorexia.
Amphetamines and precursors to norepinephrine can minimize damage and improve cognitive function as well. Cholinesterase inhibitors can be used as a first-line agent in memory impairment, although there is no evidence they work in TBI. Selective serotonin reuptake inhibitors (SSRIs) and other similar antidepressants are commonly used in patients with depression and anxiety. Antidepressants are also used in low doses to control chronic agitation and aggression. Propranolol has also been shown to improve aggression. Care must be taken as it can result in hypotension and bradycardia. Mood stabilizers such as anticonvulsants are routinely used in the treatment of post-TBI lability, impulsivity, and/or disinhibition (12). First-generation antipsychotics such as haloperidol should be avoided because they have been shown to cause neuronal damage and decreased neuroplasticity.
Several oral medications are used to control spasticity and tone in TBI. Dantrolene acts at the sarcoplasmic reticulum to inhibit calcium activity; however, it can negatively affect the liver, so care must be taken when prescribing this medication. Baclofen, which acts at GABA B receptors, is also commonly used in the treatment of spasticity. Other agents for spasticity treatment include benzodiazepines, tizanidine, and clonidine. Oral medications, other than dantrolene, cause increased somnolence, which limit their use in TBI as they can slow recovery.
Static and dynamic splinting devices are commonly used to control spasticity. In combination with passive ROM and stretching therapies, they lengthen affected muscles and tendons. Superficial and deep heat, electrical stimulation, and cryotherapy are also used in concert with medication and splinting to control spasticity.
Chemodenervation can provide local spasticity management without systemic side effects. Phenol used in nerve blocks causes denaturation of the nerve. Botulinum toxin injection into an affected muscle can also provide good spasticity control in adjunct to physical modalities and splinting.
DIAGNOSTIC TESTING
Routine laboratory work, including monitoring of electrolytes, is important in inpatient neurorehabilitation as endocrine abnormalities, such as diabetes insipidus, are common after brain injury. Prealbumin levels and liver function should be followed to monitor nutrition status with the goal to achieve a positive nitrogen balance to meet the increased metabolic demand following brain injury.
CT scan is the standard for initial evaluation for patients with moderate to severe TBI. It can provide early detection of bleeds, contusions, and other mass lesions and can dictate neurosurgical intervention. CT is also used to monitor progression of the brain injury and to evaluate for complications such as rebleed or hydrocephalus. CT is better than MRI when used to evaluate skull fractures. MRI is more useful when evaluating for extent and location of axonal injury, imaging the posterior fossa, and visualizing cortical and subcortical hemorrhages and edema.
Somatosensory evoked potential (SSEP) is used in severe TBI to predict survival; it can also evaluate coma and vegetative state. SSEP records transmission from the scalp after stimulation of peripheral or mixed nerves. EEG (electroencephalography) can detect injury severity and depth of coma; it is also used in evaluation of seizures. Continuous EEG can evaluate for subclinical seizures that are associated with a poorer outcome.
ETHICAL ISSUES
Bioethics plays an important role in the recovery of persons with TBI. Table 14.4 describes six terms that play a role in ethical issues associated with TBI. Capacity is the person’s ability to make his or her own medical decisions. It is not static and can be determined on a decision-to-decision basis. A patient may be able to make simple decisions but may not be able to make a more complex medical decision. Capacity is based on many cognitive factors including alertness, communication, orientation, and understanding and manipulating relevant medical information in regard to the consequences of medical decision making. Competency is a legal term and can only be formally determined by the legal system. If a person is deemed incompetent by a court, it may affect other legal decisions outside of the medical realm including the right to vote, marry, and enter into other legally binding agreements. At times, providers struggle with the balance of beneficence (doing good for the patient) versus autonomy (patient making an informed and voluntary decision). In the patient with TBI, these bioethical principles may be at odds. A patient with poor executive functioning and awareness may make a decision that can harm himself or herself, so it is up to the TBI provider to explain treatment options in a manner that the person with TBI can understand. At times, only the social support system can convince a person with TBI to follow the appropriate decision. Additionally, it is the duty of the physiatrist to practice with nonmaleficence (do no harm to the patient). This can be accomplished by minimizing risks of procedures or adverse effects of medications. Lastly, a physiatrist must acknowledge that there are various stakeholders in the rehabilitation unit. It is important to balance the leverage of those stakeholders with the principle of justice. Persons with TBI are a vulnerable population; the physiatrist must advocate for them by allowing equal access to care.
TABLE 14.4 Ethical Principles in TBI Rehabilitation
Capacity | A person’s ability to make his or her own decisions |
Competence | A person’s mental capacity to participate in legal matters |
Beneficence | A provider performs an action to benefit the patient |
Autonomy | A person’s ability to make informed and voluntary decisions |
Nonmaleficence | To not intentionally harm or injure a person |
Justice | Fair and equal treatment for all |
At times, the physiatrist is faced with the difficult task of discussing end-of-life care with social support systems. Although persons with TBI can have vastly different outcomes, sometimes the outcome is death. It is important to incorporate the services of mental health and/or hospice providers when discussing end-of-life matters. A team approach will provide the family with an understanding of the process. If the person with TBI has an advanced directive dictating end-of-life care or his or her wishes, this will make the conversation and decision easier on family members. Approaching the conversation with empathy and compassion is paramount. TBI providers must allow family members to grieve and make decisions in their own time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




