Chapter 152 Metastatic Disease About the Knee
Evaluation and Surgical Treatment
According to the latest cancer statistics, at least 1,368,030 new cancer cases are diagnosed annually in the United States.12 Those cancers with a propensity for bone metastases rank among the most commonly diagnosed types of new cancers. Both breast and prostate cancer rank first in numbers of new cases for women and men, respectively. Lung cancer ranks second for men and women, kidney cancer ranks seventh among men, and thyroid cancer ranks eighth among women.
Metastatic disease to bone occurs less commonly about the knee than it does in more proximal femoral sites, but the distal femur is not an uncommon site of metastatic lesions or pathologic fractures.8 Metastatic disease is particularly rare distal to the knee joint, just as it is distal to the elbow in the upper extremity. Metastatic disease to the tibia has been estimated to account for only 4% of pathologic fractures, although lesions in this location are probably more frequent.10,13 Metastatic involvement in the patella is rare but has been reported.14,17 In part, the less frequent involvement by metastatic disease in these sites has presented a challenge to its orthopedic management because of the paucity of literature available on which to base guidelines.
In addition, the relatively distal location in the extremity presents its own challenges for diagnosis and treatment. The distal femur and proximal tibia are comprised predominantly of cancellous metaphyseal bone, so lesions may become large before they are evident radiographically. The occurrence of metastatic disease to bone or synovium following total knee replacement is a rare occurrence but should be kept in mind as a potential source of pain after total knee replacement.1,4,6,20
Treatment Principles
The corollary to the minimum survival rule is that the operation should result in a stable reconstruction that allows immediate weight bearing and durability for the patient’s shortened life span. The improved function and pain relief provided by an immediately stable construct will translate into improved quality of life over the period of limited survival. Although a cemented endoprosthetic reconstruction will usually accomplish this goal, the same may often be achieved by internal fixation. In a fracture situation, supplementing internal fixation with bone cement will often allow immediate weight-bearing, but fixation durability relies on fracture healing. If the patient survives long enough and the fracture fails to heal, the construct will likely fail. The healing of pathologic fractures is slowed by local disease progression and by radiotherapy, but is also closely related to the underlying disease process and expected survival. Patients with metastatic lung cancer, for example, rarely survive longer than 3 to 6 months, so their fractures rarely heal. Some fractures in patients with breast carcinoma metastases will heal, given appropriate treatment, because many will live longer than 6 months. Even the best reported healing rate for pathologic fractures, which occurred for multiple myeloma, was only 67%.7
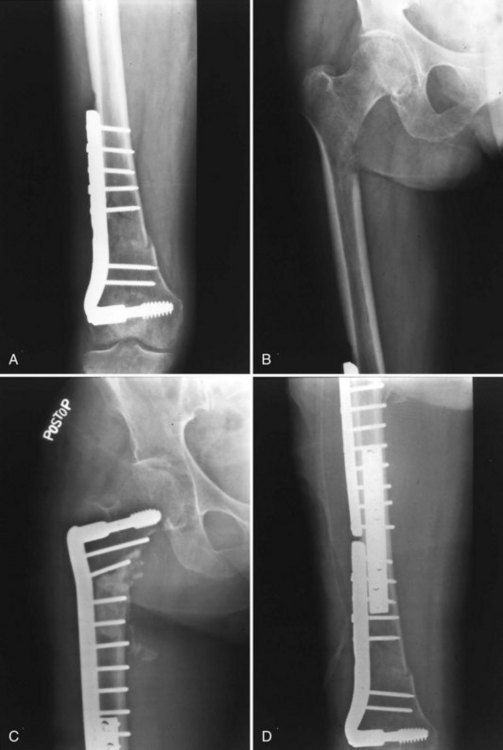
One of the most important but often neglected axioms for orthopedic treatment of metastatic disease is the protection of the entire bone proximal and distal to the lesion (Fig. 152-1). This is especially important to address preexisting bone lesions that may progress to weaken the bone or cause fracture, but it is also recommended prophylactically to address new sites that may arise as the disease progresses. With fixation of the distal femur with a retrograde intramedullary nail or long-stemmed femoral total knee component, the intertrochanteric region and/or femoral neck remain unprotected and are frequent sites of metastases. Failure to consider proximal femoral impending pathologic fractures may result in pathologic fracture proximal to an internal fixation or prosthetic device. Prior to operative intervention, radiographs of the entire affected bone should be reviewed.
Finally, radiotherapy should be used postoperatively to protect the entire instrumented region of the bone. When intramedullary reaming is done, the entire bone should receive irradiation. Failure to irradiate postoperatively leads to a higher rate of implant failure and lower functional benefit overall.18 Consultation should be obtained with a radiation oncologist preoperatively or in the early postoperative course for this purpose. When incisions are laterally placed, they can often be avoided by anteroposterior-posteroanterior (AP-PA) radiation treatment, but incisions within the actual radiation field should usually be allowed to heal completely before initiating radiotherapy.
Evaluation
For patients without an established diagnosis, a comprehensive history should seek any history of prior biopsies or tumor excisions, however remote (Table 152-1). Physical examination should also be comprehensive, including evaluation of the breast or prostate, abdomen, and thyroid and may be best performed by an internist or oncologist. Lymphadenopathy should also be sought. Serologic examination, including serum and urine protein electrophoresis for multiple myeloma and prostate-specific antigen in men, should be done along with checking the serum calcium level to identify hypercalcemia. Radiographic evaluation, including chest radiography and mammography, when appropriate, should be done initially, followed by chest computed tomography (CT), and abdominal-pelvic evaluation by CT or ultrasound. This approach will reveal the diagnosis in approximately 85% of patients.16
Table 152-1 Key Elements in Evaluation of Patient With Metastatic Disease of Unknown Primary
| Key Element | Features of Metastatic Disease to Evaluate |
|---|---|
| History | Carcinomas, biopsies; purpose of hysterectomy, TURP |
| Physical examination | Breast or prostate examination, thyroid, abdominal masses, lymphadenopathy |
| Laboratory evaluation | CBC, SPEP-UPEP, PSA, LDH, calcium levels |
| Radiographic evaluation | Chest radiograph, radiographs of entire affected bone |
| Total skeleton 99Tc bone scan | |
| CT scanning of chest, abdomen, pelvis |
CBC, Complete blood count; LDH, lactate dehydrogenase; PSA, prostate-specific antigen; SPEP, serum protein electrophoresis; 99Tc, technetium-99; TURP, transurethral resection of prostate; UPEP, urine protein electrophoresis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree