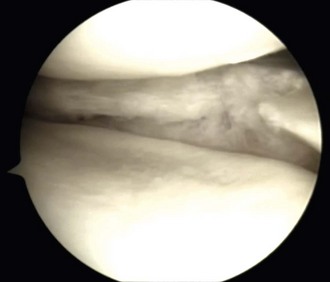
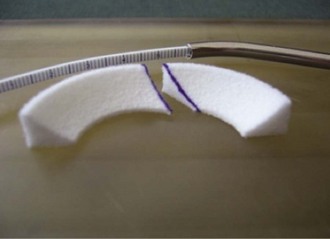
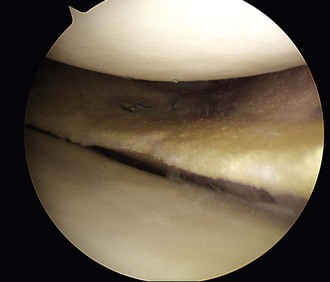
Chapter 59 Meniscal regeneration appears to require the physical presence of a scaffold to encourage successful migration and colonization with precursor cells and vessels, eventually leading to the formation of organized meniscal tissue.1,2 The key inclusion criteria are as follows: 1. Irreparable medial or lateral meniscal tear or partial meniscus loss, with intact rim. Most important, the synthetic meniscus substitute is not intended for the treatment of total or subtotal meniscus defects. Ideally, the defect length should be limited to 5 to 6 cm. 2. Skeletally mature male or female patients. 3. Patients aged 16 to 50 years. 4. Stable knee joint or knee joint stabilization procedure within 12 weeks of index procedure. 5. International Cartilage Repair Society (ICRS) classification of 3. 6. Patient willing and able to give consent to participate in the clinical study, follow rehabilitation protocol, and attend or undergo all follow-up visits and procedures. The key exclusion criteria are as follows: The specific steps of the procedure are outlined in Box 59-1. Preparation of the damaged meniscus includes surgical debridement and removal of all pathologic tissue and ensuring that the resulting defect site extends into the vascularized red-on-red or red-on-white zone of the damaged portion of the meniscus. Lesions situated farther away from the synovial border are known to have only very limited healing potential and therefore should be excluded from this type of meniscoplasty. For enhancement of healing, the meniscal rim may be punctured to create vascular access channels. Gentle rasping of the synovial lining may further stimulate meniscal healing (Fig. 59-1). The meniscal defect should be measured along the curvature of its inner edge using the accompanying specially designed meniscal ruler and meniscal ruler guide. Actifit is then measured and with a scalpel is cut to fit in such a place and manner as to ensure that sterility is maintained at all times. To allow for shrinkage caused by suturing of the spongelike material and to ensure a snug optimal fit into the prepared defect, oversizing of the length by 10% is advised (3 mm for defects smaller than 3 cm and 5 mm for defects 3 cm or larger). To achieve a perfect fit of the scaffold with the native meniscus at the anterior junction, the anterior side should be cut at an oblique angle of 30 to 45 degrees (Fig. 59-2). Although the Actifit material is strong and flexible, the device should be handled with care and manipulated with a blunt-nosed grasper, such as the Acufex Grasper Tissue Tensioner (Smith & Nephew). Marking the cranial and caudal meniscal scaffold surface with a sterile marking pen avoids positioning problems. The Actifit device should be clamped at the posterior part of the scaffold and placed into the knee joint through the anteromedial or anterolateral portal. To ensure a good initial position of the meniscal scaffold and facilitate further fixation, the surgeon may place a vertical holding suture in the native meniscus tissue to bring Actifit through the eye of this holding suture. To enhance the healing response, bone marrow aspiration can be performed from the notch area and directly applied on the dry scaffold after implantation. Figure 59-3 depicts a scaffold 1 year after implantation showing full peripheral integration to the meniscus rim.
Meniscus Substitution
The European Perspective on Scaffolds, Allografts, and Prosthetic Implants
Meniscus Scaffolds for the Treatment of Partial Meniscus Defects
Indications
Surgical Technique
Specific Steps
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Meniscus Substitution: The European Perspective on Scaffolds, Allografts, and Prosthetic Implants