Fig. 27.1
Arthroscopic images of a 17 years old female soccer player with a subtotal partial meniscal defect (a) Polyurethane scaffold implanted to the meniscal rim, (b) the scaffold is completely healed and integrated to the native tissues 12 months after implantation (PUS polyurethane scaffold, NM native meniscus, F femur, T tibia, RM regenerated meniscus)
In 1992, Stone et al. introduced the first meniscal scaffold, composed of bovine Achilles-tendon type I collagen fibers, to replace partial meniscal defects [21]. After initial investigations in canines, preliminary data obtained from human studies were published in 1997, reporting satisfactory results in alleviating pain and ingrowth of host meniscal tissue [22]. Currently there are two major, tissue-engineered scaffold types in clinical use: collagen meniscal scaffold (CMI, Menaflex, Ivy Sports Medicine GmbH., Grafelfing, Germany) and polyurethane meniscal scaffold (Actifit, Orteq Bioengineering Ltd., London, UK) [2, 3, 6, 7, 23, 24]. The former one, CMI-Menaflex, was cleared for use in Europe in 2000 for substituting the medial meniscus and in 2006 for the lateral meniscus whereas Actifit was approved for clinical use in Europe, in 2008, for substituting both menisci [3, 6]. The aim of this chapter is to review the current concepts about meniscus scaffolds and discuss possible future improvements in this area.
27.2 Meniscal Scaffolds
Meniscus scaffolds are developed to overcome devastating outcomes of partial meniscectomies (Table 27.1). These scaffolds aim to provide a structure, which allows ingrowth of meniscus-like tissue from the host environment. An ideal scaffold should be easily available with various sizes fitting for individual patients. It should not have any toxic degradation products and provide good stability. There should be no risk of disease transmission and the material which the scaffold is made up of should be biocompatible. The porosity of the scaffold should be high allowing the intrusion of nutrients. Promoting cell differentiation and proliferation or cell migration along with being biodegradable are other important properties of an ideal scaffold [10].
Table 27.1
Indications and contraindications of meniscus scaffolds
Meniscus scaffolds | |
|---|---|
Indications | Contraindications |
Irreparable meniscus tears >25–50% | Infection |
Pain and swelling due to partial meniscectomy | Known allergy to the ingredient of the scaffold |
Loss of meniscal tissue >25–50% | Uncorrected malalignment of the knee |
Chronic posttraumatic meniscal tears | Accompanying posterior cruciate insufficiency |
Intact anterior and posterior horns plus presence of circumferential intact meniscal rim | Untreated, high-grade cartilage lesion within the knee (International Cartilage Repair Society Score—ICRS >2) |
Osteonecrosis of the knee | |
Posterior knee instability | |
Immunologic disorders | |
Total meniscectomy | |
Inflammatory arthritis | |
Body mass index >35 | |
Immature patients | |
Pregnancy | |
In order to establish the ideal scaffold, numerous biomaterials have been studied so far. These could be evaluated in four main groups: Extracellular Matrix (ECM) component scaffolds, synthetic polymer scaffolds, hydrogels, and tissue-derived scaffolds (Table 27.2) [13]. Among various scaffold options, CMI, which is an ECM Component scaffold and Polyurethane scaffold, a synthetic polymer, are commonly used with the aim of partial meniscal substitution.
Table 27.2
Biomaterials, being used as scaffolds
Meniscal scaffolds | Extracellular matrix component scaffolds | Collagen meniscus implant |
Hyaluronan and gelatine | ||
Collagen-glucoseaminoglycan | ||
Synthetic polymer scaffolds | Polyurethane | |
Polylactic acid | ||
Polyglycolic acid | ||
Polycaprolactone | ||
Polylactic co-glycolic acid | ||
Poly L lactic acid-poly p-dioxanone | ||
Hydrogels | Alginate | |
Poly N isopropyl acrylamide | ||
Polyvinyl alcohol | ||
Tissue-derived scaffolds | Silk | |
Intestinal submucosa | ||
Decellularized tissue | ||
Periosteal tissue | ||
Perichondral tissue | ||
Bacterial cellulose |
27.2.1 Collagen Meniscus Implant (CMI)
As CMI was available since nineties, it has been extensively studied. After initial in vitro studies by Rodkey et al. [25] numerous animal studies were initiated and no apparent negative effects were observed. The newly generated tissue was grossly and histologically similar to the native meniscus. Further human studies confirmed that CMI could be used to reconstruct the lost meniscus tissue and improve functional outcomes [26]. CMI may be used for both medial and lateral meniscus defects.
27.2.1.1 Mechanical and Biological Properties of CMI
The scaffold is composed of purified type I collagen isolated from bovine Achilles tendon without any noncollagenous materials and lipids. The collagen fibers are enriched with glycosaminoglycans, including chondroitin sulfate and hyaluronic acid. The fibers are then cross-linked to improve stability and ease implantation and handling. Sterilization is performed with gamma radiation [21]. The implant has no carcinogenicity, pyrogenicity, or cytotoxicity [27]. Biopsies, in second-look arthroscopies, have proved extensive resorption of the scaffold in 12–18 months and ingrowth of newly formed tissue, replacing the scaffold. The implant promoted migration of fibrochondrocytes into the scaffold, establishing a new functional tissue matrix [6, 7, 24, 28, 29].
27.2.1.2 Results of CMI
In a study with a large number of patients, with either acute or chronic meniscal injury, CMI was found to improve the clinical results in the chronic injury group providing a greater recovery of function whereas it was not that useful for patients with acute injury [26]. In a recent study by Zaffagnini et al. [30], long-term clinical, radiological, and magnetic resonance imaging (MRI) results of CMI were compared with partial medial meniscectomy. Significant improvements in pain, activity level and radiological outcomes were recorded in the CMI group at a minimum 10 years follow-up when compared with the partial meniscectomy group. One of the most remarkable findings of the study was that the improvements in the International Knee Documentation Committee (IKDC), Tegner, Short Form-36, and visual analogue scale scores (VAS) were significant; however, there were no difference in Lysholm and Yulish scores. Bulgheroni et al. [11] reported clinical, radiological, and MRI results of CMI application at 5 years. In this study, there was a significant increase in both Lysholm and Tegner scores and no further degeneration of the chondral surfaces was seen after CMI application. The new tissue was reported to have no negative effects. In another series of 22 patients, followed-up for a minimum of 10 years, Monllau et al. [31] reported significant improvement in functional and pain scores at 1 year and the functional status of the patients was the same in the last controls. There were no reported complications and the failure rate was 8%.
CMI is found to be useful for treating partial meniscus defects in numerous studies. After implantation, the scaffold is invaded by cells and undergoes resorption. Subsequent formation of a regenerated tissue is seen in majority of cases. The new tissue matures over time but the size is usually smaller than the normal meniscus and the shape is irregular. Key factor for achieving a satisfactory result is selecting the suitable patient.
27.2.1.3 Combined Surgeries for Associated Knee Pathologies
Implantation of CMI does not preclude accompanying surgical procedures in most cases and combined surgical procedures are rather common. Anterior cruciate ligament (ACL) reconstruction for ACL deficient knees improves meniscus healing and thus combined reconstruction of both structures is recommended. Reconstruction may be performed in one stage or if concurrent procedures are preferred, CMI implantation should be performed in the first stage, as it is more difficult to implant CMI after ACL reconstruction, due to increased tightness of the knee. Varus or valgus malalignment deformities should be corrected either with or prior to CMI implantation (Fig. 27.2). Chondral lesions detected within the knee should also be managed with the appropriate treatment modality such as microfracture, mosaicplasty, or osteochondral transplantation. Main concern about management of chondral lesions is about timing as an altered, rough chondral lesion is considered as detrimental for the newly implanted CMI [11, 32, 33].
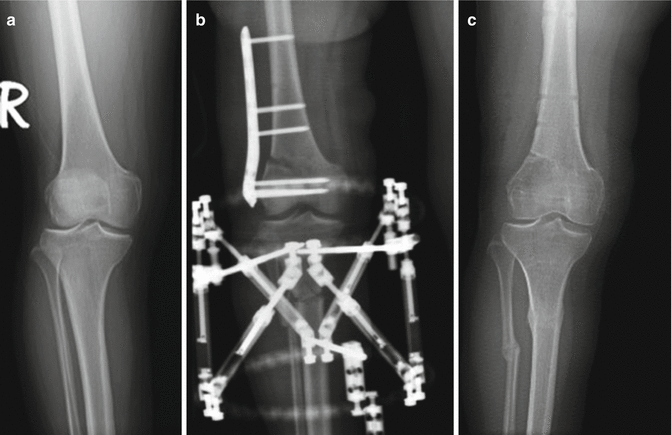

Fig. 27.2
X-rays of the same patient. The malalignment in the limb was corrected simultaneously (a) Preoperative x-ray, (b) X-ray showing the femoral and the tibial osteotomies, (c) X-ray at the time of second-look arthroscopy
Though, experience with lateral CMI is lower when compared to medial CMI, and clinical results are similar to those obtained with the medial implant. Whether medial or lateral, swelling and residual compartmental pain are the most commonly reported complications after CMI implantation [27].
27.2.2 Polyurethane Meniscus Scaffold (Actifit)
After years of clinical experience with CMI, reduction in size and alteration of morphology were two most common findings, leading to further research, in search for a more stable implant in regard to size and morphology [3]. Polyurethane meniscus scaffold is made of aliphatic polyurethane, providing its optimal biocompatibility and mechanical strength [34]. Polyurethane meniscus scaffolds were approved for use in Europe in only 2008, thus the clinical experience regarding the implant is still limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






