Materials in Hip Surgery
Mechanical Properties That Influence Design and Performance of Ceramic Hip Bearings
Ian C. Clarke, Giuseppe Pezzotti and Nobuhiko Sugano
Key Points
• The AMC ceramic now provides a 60% greater strength alternative to ALX.
• In terms of ceramic ball designs, the short-neck ball is twice as strong as the long-neck design.
• m-ZR ceramic balls with polyethylene cups are still in use in the United States.
Introduction
Properties of Ceramic Implants
From a materials science point of view, the broad classification of ceramics includes all nonmetallic and nonorganic materials. For orthopedic implants, this includes forms of pure carbon and silicon along with carbides, oxides, and nitrides of base metals such as aluminum, magnesium, and zirconium. Ceramic materials are typically suitable for tasks that would be very difficult for survival of plastic and metal bearings. For example, pure alumina is an inert ceramic that is classed as 10 on the Mohr hardness scale, where the hardest known material diamond is ranked at Mohr 11. This property of high hardness makes a ceramic bearing extremely resistant to third-body abrasive wear. Alumina ceramic also has the highest rigidity of any implant material. Its elastic modulus (450 GPa) represents twice the stiffness of the nearest metal alloy (CoCr: 210 Ga). Thus ceramics are called into play when adverse combinations of temperature, pressure, stress, lubrication, and abrasion resistance are required. Thus the benefit of ceramic for total hip replacement (THR) is primarily that of a bearing that is dimensionally stable and chemically inert and possesses exceptionally high wear resistance.
Oxide ceramics may include two or more types of atoms. Oxygen ions typically are closely packed around the metal ions and thus shield reactive metals from the external environment. The major oxide ceramic used over a 40-year history in Europe and elsewhere has been pure alumina (Fig. 7-1).1 Alumina is the oxide of the base metal aluminum; it has been extensively studied as an implant material and ranks highest in terms of physical and chemical inertness and biological compatibility. The strength of alumina implants has been steadily increasing (Table 7-1). The mating of ceramic with metal components has generally involved taper-locking geometries to smooth the transfer of interfacial stresses. Therefore, this is a critical component of design, materials selection, and quality control. Note that mating a ceramic liner with a flexible polyethylene sheath represents the most extreme example of adverse modulus and strength mismatches.2 This will be discussed in a later section.
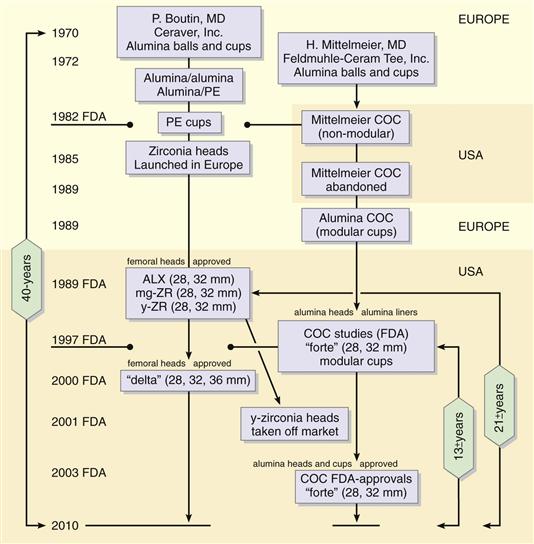
Figure 7-1 The 40-year history of ceramic hips.
Table 7-1
Mechanical and Physical Properties of Ceramics Used in Hip Bearings
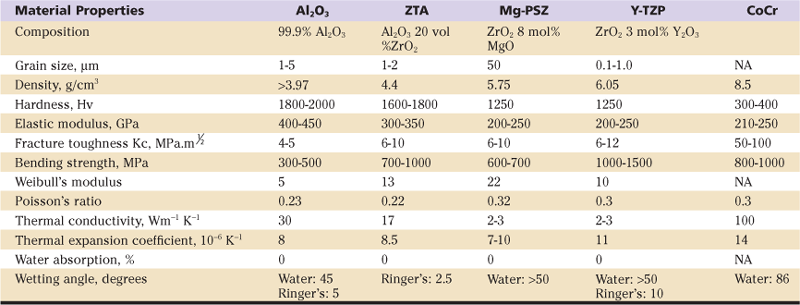
Improved processing of ceramic implants has included as most relevant a “hipping” process by which fully dense alumina implants could be obtained while grain growth was limited. This was an important limitation because abnormal shapes or sizes of ceramic grains would act as crack sites and so could lower the inherent strength of the implant. Thus hot-isostatic pressing has been in use since 1975 to 1977. The introduction of the proof test significantly improved the reliability of these components. Before its introduction, the only method of strength testing involved loading of components to fracture, so that only 2% to 3% of implants underwent testing. Now 100% of ceramic implants are proof-tested at stresses above physiologic levels before leaving the factory.3
The next well-known structural ceramic is zirconia. Unlike pure alumina (ALX), zirconia (ZR) can exist in several phases and so is called polymorphic. Zirconia implant grades are alloyed with yttria (y-ZR) or magnesia (m-ZR). The y-ZR ceramic is manufactured to include predominantly the tetragonal phase. Under certain conditions, it can transform with some volume expansion into the monoclinic phase. More will be said of this “toughening” concept later. Carbon implants have also been made available as various implant coatings, and even with bearing inserts made of industrial diamond.
Ceramic Total Hip Replacement Developments in the United States
The pioneering Mittelmeier ceramic THR (Autophor, Xenophor) represents a flawed experience in the United States (see Fig. 7-1). The U.S. Food and Drug Administration (FDA) gave premarket approval (PMA) in November 2002 to Richards Surgical (now Smith & Nephew, Memphis, Tenn). The first clinical studies noted major problems from both stem and cup loosening, and revisions appeared with at least a 20% incidence.4–6 Approximately 3500 Mittelmeier THRs were implanted in the United States prior to their voluntary removal from the market. However, this first ceramic THR experience did pave the way for the FDA down-classification of alumina implants with the approval of ceramic/polyethylene combinations (CPE) in 1989 (see Fig. 7-1). All-ceramic bearings (ALX/ALX; 28 and 32 mm) were granted approval some 14 years later. Thus three key FDA actions controlling the sale of ceramic hips occurred in 1982, 1989, and 2003 (see Fig. 7-1). Marketing approvals also included the ceramic called zirconia in both magnesia-stabilized (m-ZR) and yttria-stabilized (y-ZR) forms.7
The introduction of ceramic liners with modular acetabular shells in 1989 represented another European innovation (see Fig. 7-1). Three companies launched FDA-monitored clinical studies a decade later with this modular cup design,8,9 and FDA approvals to market began in January 2003. Note that because of FDA marketing requirements, these ceramic-on-ceramic (COC) developments were exclusive to products from one vendor.
The year 2000 saw the introduction of a composite alumina ceramic that had small zirconia grains interspersed between larger alumina grains. This ceramic has been referred to as an alumina matrix composite (AMC).10 At the present time, the FDA has approved only AMC balls for use with polyethylene (PE) cups (diameter, 28 to 44 mm), although AMC balls can be used with either ALX or AMC cups in Asia and Europe.11
Strength, Toughness, and Safety Issues in Ceramic Implants
The strength of a ceramic femoral ball is dependent on many material parameters, but primarily the design of the metal trunnion. The standard strength test of ceramic balls, the compressive burst test, is used in all applications to the FDA (ASTM F2345).12–14 For example, it is well known that the short-neck ball design is approximately 50% stronger than the long-neck design (Fig. 7-2) of the same diameter, which is counterintuitive because the long-neck ball has greater wall thickness at a critical stress point (see Fig. 7-2, insert; TL). The reasons why long-neck ceramic femoral heads in fact are weaker than short-neck heads are as follows: (1) short-neck heads engage the taper over a larger surface area, thereby reducing stress; (2) in the long-neck head, stress transfer occurs lower in the ball taper in an area that is weaker; and (3) the long-neck head has a longer lever arm and creates proportionately higher deformation of the metal neck in the taper and thus greater stress.
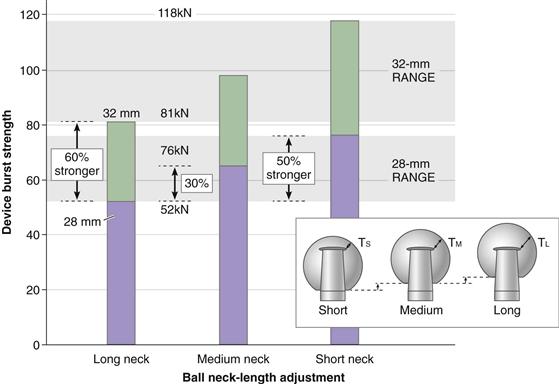
Figure 7-2 Range of burst strengths with varied neck lengths and diameters of ALX balls. Inset depicts the wall thickness (T) increasing with longer neck lengths (inset cartoon depicts wall thicknesses; TS< TM<TL). (Biolox-Forte data courtesy of CeramTec AG, Plochingen, Germany.)
By FDA guidelines, average burst strength should exceed 46 kN (approximately 10,000 lb), with no individual part failing at loads below 20 kN (5000 lb).15 It is obvious that 32-mm balls are much stronger than 28-mm balls. For the critical long-neck design, the 32-mm ALX ball is approximately 60% stronger than the 28-mm ball (see Fig. 7-2). In this regard, the CeramTec database showed that the fracture incidence for 32-mm balls was 0.007% compared with 0.03% risk for 28-mm balls (i.e., a fourfold reduction in risk) (see Fig. 7-1).
The most direct path to increasing the strength and reliability of a ceramic lies in increased fracture toughness, as observed with zirconia (m-ZR, y-ZR) or zirconia-reinforced alumina (AMC; see later). Yttria-stabilized, zirconia polycrystalline ceramic (y-ZR) represents an extremely strong and fatigue-resistant material. This ceramic has the ability to transform from the as-manufactured tetragonal phase into the stable monoclinic phase. The tetragonal-to-monoclinic transformation produces a net 4% volume expansion of the zirconia grains. Thus, when a crack tries to propagate through a matrix composed of tetragonal grains, the sudden loss of matrix constraint allows spontaneous expansion from the tetragonal to the monoclinic phase. Resulting compressive stresses inhibit growth of the crack; this effect is termed transformation toughening. However, this could become a negative effect if transformation takes place spontaneously on the bearing surface as the result of the resulting increase in surface roughness.
Implant designers initially welcomed the introduction of zirconia ceramics, given the prospect of increased reliability, strength, and toughness compared with ALX (see Table 7-1). The zirconia saw widespread use in CPE bearings and in a few cases with y-ZR/y-ZR and y-ZR/ALX combinations.16 However, the emergent problem observed with zirconia components partially stabilized with yttrium oxide (y-ZR) was lack of inherent stability. This “metastability” resulted in significant and unforeseen degradation in mechanical properties in vivo when implants were manufactured under suboptimal conditions. Thus an unfortunate process change by one manufacturer in France resulted in a very high incidence of y-ZR ball fractures. Subsequent product recalls ended the life cycle of y-ZR ceramic balls by the year 2001 (see Fig. 7-1). Note that Mg-stabilized zirconia balls (m-ZR) are still in use.7,17
A ceramic composite material was developed to combine the superior bearing properties of alumina while exploiting the superior strength and toughness of zirconia (Table 7-2). In this concept, the toughening effect of zirconia is used to enhance the strength of alumina (see Table 7-2). However, zirconia also reduces the hardness of the composite ceramic. This can be alleviated by alloying alumina with chromium oxide, creating a solid solution within the alumina matrix. This distribution of chromium inside the alumina lattice gives components a purple hue. The hardness of the resulting material is thus greater than that of the y-ZR ceramic, but still is not as great as that of alumina (see Tables 7-1 and 7-2). However, in terms of safety, the toughness of zirconia-reinforced alumina (AMC) is increased approximately 50% to 60% compared with that of pure alumina (see Table 7-2). In terms of the critical long-neck design, the burst test showed that AMC balls achieved 60% higher loads than alumina (Fig. 7-3). Here, the long-neck 28-mm AMC ball was as strong as the 36-mm ALX. Thus from the point of view of the AMC ceramic, the alumina phase externally provided the ideal bearing surface, while the zirconia phase internally contributed to strength and toughness.18
Table 7-2
Comparison of Hardness, Strength, and Toughness Properties for Biolox-Forte (ALX) and Biolox-Delta (AMC)
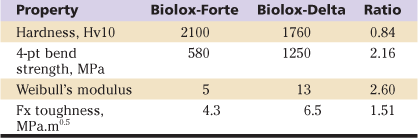
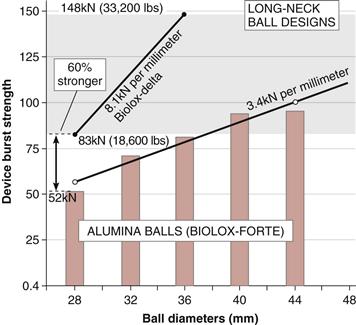
Figure 7-3 Burst strengths with varied diameters of long-neck (LN) ball design. Strength of two diameters of AMC balls (28 mm; 36 mm) indicated for comparison. (Data courtesy of CeramTec AG, Plochingen, Germany.)
Ceramic Tribological Properties
Mechanics in the Tribology of Ceramic Cups
The hip simulator has been the dominant tool for assessing wear in THR bearings (science of tribology). The Standard Procedures specified by the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) for hip simulators provide guidelines for testing actual implants; however, these were developed specifically for metal and polyethylene bearings.19–22 Existing guidelines omit important test details relevant to ceramic tribology and provide no specific warnings with regard to interpreting ceramic wear phenomena (as discussed in later sections).11,23-31 In addition, available hip standards specify only what will be termed the standard simulator test (ASTM F1714; ISO 14242-3). In this mode, a predominantly compressive load is applied during both stance and swing phases (Table 7-3). Little or no separation of bearing surfaces is noted in the standard test mode, and the wear zone typically never crosses the cup rim (Fig. 7-4). This is a very conservative test under ideal lubrication conditions. It is likely that more adverse conditions are commonly present in the patient’s hip joint (discussed in later sections). In contrast, the microseparation test mode permits the femoral head to slightly subluxate from the cup during the swing phase (see Table 7-3) under the action of an applied distraction (“negative”) load. Thus during heel-strike and toe-off load impacts, the rim of the acetabular liner is free to impact on the rotating femoral head. This creates stripe wear similar to that seen on retrieved ceramic bearings.32,33 This “severe” microseparation test has little likelihood of being confounded by the proteinaceous effects of serum lubricants.
Table 7-3
Comparison of Standard (STD) and Microseparation (MSX) Test Modes
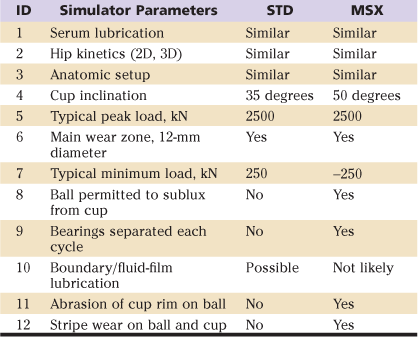
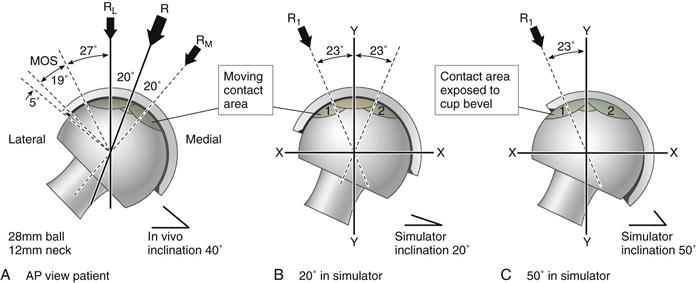
Figure 7-4 Contact areas under the path of the resultant load (R). These are compared for cups inclined at 40-degrees in the patient (A) and 20 degrees (B), and 50 degrees (C) in the hip simulator (i.e., representative of standard and microseparation tests, respectively).57
During laboratory wear testing, the acetabular cup is typically oriented with 40 degrees of lateral inclination. It is assumed that the resultant load (R) is located 20 degrees medial to the vertical and oscillates ±20 degrees, as indicated (see Fig. 7-4A). For a 12-mm contact zone (28-mm ceramic ball), cyclical translation to the medial side (see Fig. 7-4A, RM) occurs into the safe zone. On the lateral side (see Fig. 7-4A, RL), translation of the contact area does not cross the bevel of the ceramic liner. In this example, a 19-degree arc represents the margin of safety. However, in the hip simulator, there is no anatomic meaning for the terms medial and lateral. The simulator’s resultant load is aligned in the vertical plane (see Fig. 7-4B). Thus the 40-degree cup inclination in the patient (see Fig. 7-4A) corresponds to a 20-degree cup angle in the simulator (see Fig. 7-4B), while the 50-degree cup position (see Fig. 7-4C) would correspond to a 70-degree inclination in the patient. In such a case, there is no margin of safety, because the contact wear zone can translate across the rim of the liner during every cycle. This is a severe but clinically relevant test. Note that no regulatory or standard guidelines have been provided for such clinically relevant microseparation test modes.
Validating Laboratory Wear Performance With Ceramic Clinical Results
Hip simulators require liters of lubricant to run the standard test of 5 million cycles, believed to represent 3 to 5 years of use in the patient.1,34 Because no ready supply of synovial fluid is available, diluted bovine serum has been the lubricant of choice for decades.35 To prove a point about water lubrication being nonphysiologic, the Peterson Tribology Laboratory ran studies with various material combinations (CoCr/PTFE, CoCr/PE, ALX/PTFE, and ALX/PE) with both water and serum lubrication. Compared with serum, water reduced ALX/PE wear rates to close to zero. With the CoCr/PE combination, wear also decreased, but less so. This phenomenon was clearly explained as an artifact of water lubrication.23,24 Thus serum proteins are necessary to promote physiologically relevant PE wear. However, as discussed later, a review of the salient clinical history reveals that confounding artifacts were introduced into laboratory studies of ceramics. In the simulator laboratories, turning wear data into clinically relevant predictions can be a major challenge. Laboratory wear studies are only as good as their predictive power. Therefore it is important to validate simulator data wherever possible using good clinical and retrieval data.
With a strong history of alumina in Europe, a review of CPE clinical performance noted that ALX balls conferred approximately 40% wear reduction compared with CoCr balls.11 However, the first warning of monoclinic transformation in the y-ZR/PE combination used in Europe came from a retrieval report on two Japanese cases in which a 20% to 30% phase change was detected.16,39 A French study also revealed 20% to 30% monoclinic transformation occurring 4 to 11 years post implantation.36,37 The ominous report at 12 years was that the short-term wear rate had quadrupled for 28-mm ZR/PE, which now was fivefold higher than the rate for the larger-diameter ALX/PE combination (Fig. 7-5B). These and other studies showed that y-ZR phase transformations could climb to over 80%.38 A clinical study of y-ZR balls (6 years’ follow-up) noted that PE wear rates showed an average 43% increase compared with CoCr balls. This zirconia series had 7% revisions, whereas CoCr revisions were reported as zero. The longer-term French study36 compared PE wear with y-ZR balls versus that with stainless steel and ALX balls.37 At 5 years’ follow-up (see Fig. 7-5A), PE wear with 32-mm stainless steel balls averaged 50% higher than with 32-mm ALX. By 12 years (see Fig. 7-5B), this disparity had increased 2.4-fold. In terms of osteolytic changes, the 18-year report40,45 revealed that the y-ZR/PE combination was now much inferior to ALX/PE.
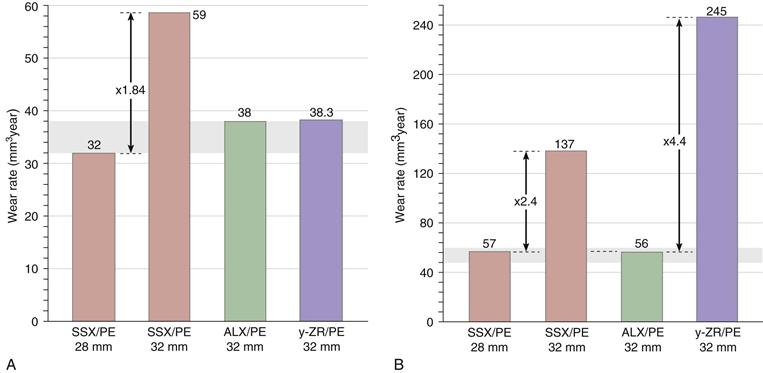
Figure 7-5 Clinical and radiographic studies of polyethylene (PE) wear with different femoral heads at (A) 5 years’ follow-up, and (B) 12 years’ follow-up.44 Note that the Y-scale in the longer-term study (Fig. 7-5B) is fourfold larger than in the short-term study (Fig. 7-6A). Also note that data from retrieval studies provide an assessment of “overall” wear.
In the simulator laboratories, various studies consistently predicted that (1) ALX/PE wear would be greater than M/PE wear,40 and (2) y-ZR/PE wear would be less than M/PE wear.40–42 It is important to note that varied ball materials have very different thermal conductivity (Fig. 7-6). For example, alumina ceramic has double the thermal conductivity of CoCr alloy (Fig. 7-7A). From a tribological perspective, the confounding factor is the use of protein-containing lubricants. In terms of wear phenomena, such increased bearing conductivity lowers lubricant temperatures and thus reduces damage to serum proteins. This one feature greatly accentuates the wear of PE liners.43 Thus in the laboratory, ALX/PE combinations generally have produced 10% more wear than CoCr/PE combinations.40,43 The Peterson Tribology Laboratory ran a comparative wear study for three types of ceramic balls using CoCr balls as controls.44 A linear increase in PE wear rates was clearly seen as thermal conductivity increased from 2.5 to 28 W•m−1•K−1 (see Fig. 7-7B; high regression coefficient, R = 0.99). This wear ranking was identical to that noted in previous laboratory data (i.e., ZR/PE MPE<ALX/PE). However, it must be noted that this simulator ranking is exactly the opposite of that predicted by long-term clinical studies (see Fig. 7-5).
MPE<ALX/PE). However, it must be noted that this simulator ranking is exactly the opposite of that predicted by long-term clinical studies (see Fig. 7-5).
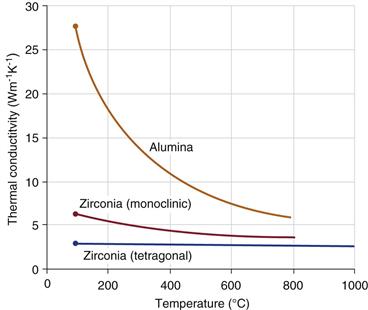
Figure 7-6 Thermal conductivity for ALX compared with tetragonal and monoclinic phases in the y-ZR ceramic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







