Chapter 28 Management of Bladder Dysfunction
Neuroanatomy and Physiology
Receptors and Neurotransmitters
The receptors active during bladder contraction are cholinergic muscarinic (M2 and M3) receptors widely distributed in the body of the bladder, trigone, bladder neck, and urethra. The M2 receptors predominate structurally in normal bladders, but the M3 receptors might be more important functionally. The cholinergic nicotinic receptors are primarily located in the striated sphincter. Adrenergic receptors are concentrated in the trigone, bladder neck, and urethra and are predominantly α1. These have recently been subdivided into α1a, α1b, and α1d. Identification of these α1 subgroups should allow increased specificity with regard to future therapeutic agents. Norepinephrine-containing nerve cells are also found in the paravesical and intramural ganglia. Some authors describe norepinephrine terminals in the striated muscle of the distal sphincter, although most would dispute this. When these cells are active, they have excitatory effects and maintain continence by contraction of the bladder neck and urethral smooth muscle. β2– and β3-adrenergic receptors are found in the bladder neck and also in the body of the bladder. These receptors are inhibitory when activated and can produce relaxation at the bladder neck on initiation of voiding and relax the bladder body to enhance storage (Figure 28-1). In humans, however, the storage role seems to be a minor one.
The main effector transmitter for contraction of the urethra is norepinephrine, via the α1 receptors. Smooth muscle relaxation is mediated by the effects of acetylcholine in the pelvic ganglia. This releases nitric oxide in the urethral wall, resulting in relaxation of urethral smooth muscle. Prostaglandins, in contrast to their effects on the detrusor, cause a relaxation of the urethral muscle. Prostaglandins have been tried in various clinical states of urinary retention but without consistent results. Serotonin appears to be an antagonist that causes urethral muscle contraction. It might be important in the production of irritable urethral symptoms. The role of estrogens on the lower urinary tract in women is confined to the modification of tissues and receptors. Apparently estrogens have no direct transmitter effects. In the brainstem and spinal cord the various transmitters described above can have a variety of inhibitory and facilitative actions, depending on their site of action. Serotonin might have inhibitory detrusor effects at the midbrain level, and uptake of serotonin might be blocked by tricyclics (used in treating nocturnal enuresis). Activation of opiate receptors in the brainstem and sacral spinal cord inhibits voiding. This might partly explain the retention of urine seen with the use of these agents.35 The pudendal motor neuron bodies are situated in the lateral border of the ventral horn of the sacral cord (Onuf’s nucleus). Serotonin and norepinephrine reuptake inhibitors prolong the effect of these agents in the synaptic cleft of Onuf’s nucleus and increase the activity of the external sphincter. (Such a dual agent [duloxetine] is currently under trial for stress incontinence in women and might be useful for some neurologic bladder conditions.15)
A complete discussion of the pharmacology of the lower urinary tract can be found in Steers.35
Peripheral Innervation
The afferent and efferent peripheral pathways include the autonomic fibers traversing the pelvic (parasympathetic) and hypogastric (sympathetic) nerves, and the somatic fibers traversing the pudendal nerves (Figure 28-2 and Table 28-1). In healthy individuals the volume of the bladder and the normal voiding reflex is routed via the afferent Aδ fibers. In pathologic states, stimulation of capsaicin or vanilloid receptor subtype 1 (VR1) (receptors expressed by unmyelinated afferents in the bladder) lead to excitation of C-afferent fibers, possibly mediating bladder dysfunction as a result of inflammatory reactions. These receptors are cation channels, expressed almost exclusively by the primary sensory neurons involved in nociception and neurogenic inflammation, and can also be activated by noxious heat and changes in pH. In suprasacral neurogenic bladder disease these capsaicin-sensitive vanilloid receptors and C-afferent fibers have a major role in the pathogenesis of hyperactivity. Intravesical capsaicin and resiniferatoxin, which block transmission through C-afferent fibers for several months, have been used experimentally to treat hyperactivity when it does not respond to the usual pharmacologic agents.20,23
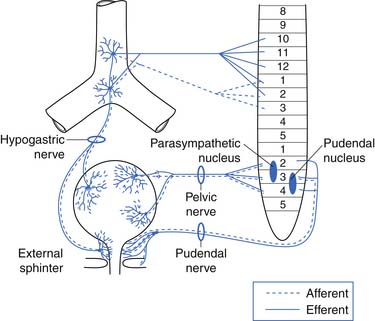
FIGURE 28-2 The parasympathetic, sympathetic, and somatic nerve supply to the bladder, urethra, and pelvic floor.
(Redrawn from Blaivas JG: Management of bladder dysfunction in multiple sclerosis, Neurology 30:12-18, 1980.)
Central Connections and Control
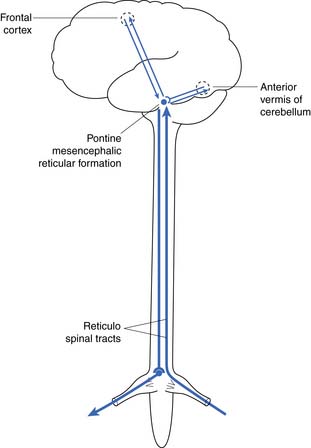
The reflex center for the bladder lies in the pons along with the other autonomic centers (Figure 28-3). Not shown in Figure 28-3 is a reflex with afferent axons originating from the bladder and synapsing on the pudendal nerve nucleus at S2, S3, and S4 (Onuf’s nucleus). This allows inhibition of pelvic floor activity during voiding. Another important reflex uses the local segmental innervation of the external sphincter with afferents from the urethra, sphincter, and pelvic floor and efferents in the pudendal nerve. Higher (voluntary) control over the pelvic floor is achieved through afferents that ascend to the sensory cortex. Descending fibers from the motor cortex synapse with the pudendal motor nucleus.6
Bladder Function
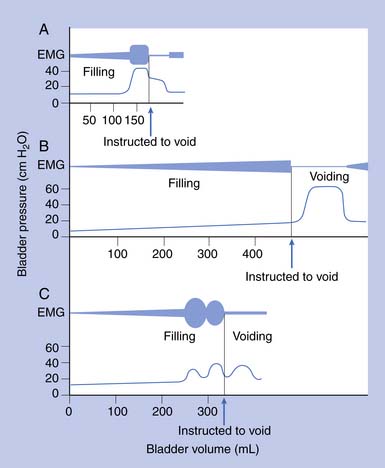
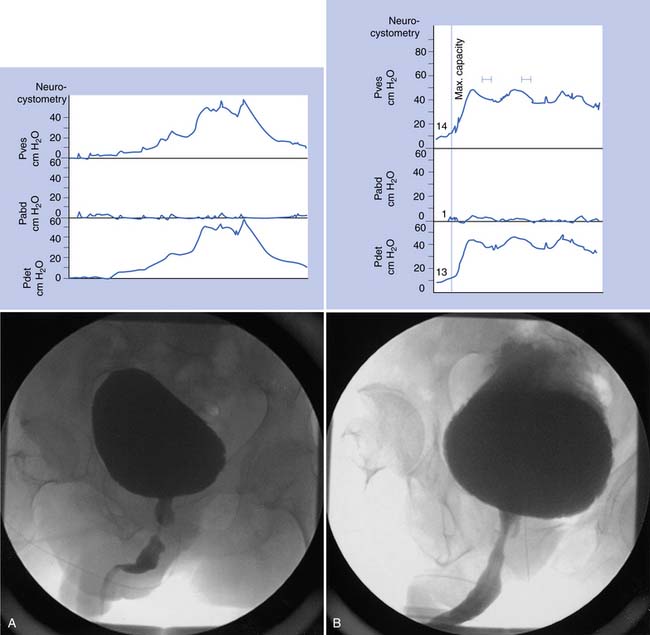
Urodynamic studies in both healthy people and those with neurologic disease have yielded clinical insights into the normal and abnormal function of the lower urinary tract over the course of life(Figure 28-4).
Elderly
Frequency, urgency, and incontinence with incomplete emptying are common in elderly persons. Urodynamic studies show that many elderly persons have bladder contractions during filling, producing frequency, urgency, and incontinence. These contractions are poorly sustained during voiding and result in incomplete evacuation. Elderly men can have prostatic obstruction, and women can have incontinence related to impaired sphincter activity or stress incontinence. In the absence of these mechanical factors, changes in bladder function in elderly persons have been ascribed to loss of cerebral control as a result of minor strokes and changes in the bladder wall caused by collagen deposition. Changes in bladder function can also result from polyuria secondary to reduced renal concentrating ability, diuretic use, lack of normal increase in antidiuretic hormone secretion at night, and mobilization of lower extremity edema during sleep.
Neurogenic Bladder Dysfunction and Classification
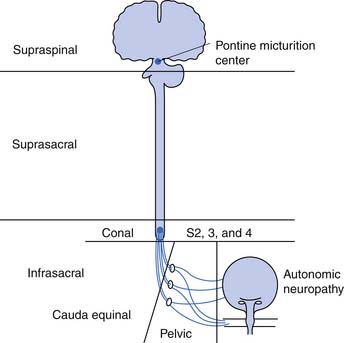
The neurogenic bladder has been classified in a variety of ways, beginning with the anatomic classification of Bors and Comarr.5 The first functional classification was based on cystometric findings, and five basic groups were described: (1) reflex, (2) uninhibited, (3) autonomous, (4) motor paralytic, and (5) sensory neurogenic bladders.31 Later a more anatomic classification system was proposed in which the neurogenic bladder was subdivided into types including supraspinal, suprasacral spinal, infrasacral, peripheral autonomic, and muscular lesions (Figure 28-5). At the same time others developed functional classifications, all of which were based on conventional urodynamic evaluations. This was an attempt to categorize the lower urinary tract according to the passive storage ability of the bladder and the activities and coordination of the detrusor and sphincter mechanisms (Table 28-2). In practice it is common to use a combination of both anatomic and functional classifications. Clinical management is based on functional changes demonstrated by urodynamic testing.
Table 28-2 Functional Classification of the Neurogenic Bladder
| Type of Failure | Bladder Factors | Outlet Factors |
|---|---|---|
| Failure to store | Hyperactivity | |
| Decreased compliance | Denervated pelvic floor | |
| Bladder neck descent | ||
| Intrinsic bladder neck sphincter failure | ||
| Failure to empty | Areflexia | |
| Hypocontractility | Detrusor–sphincter dyssynergia (striated sphincter and bladder neck) | |
| Nonrelaxing voluntary sphincter | ||
| Mechanical obstruction (benign prostatic hypertrophy or stricture) |
Evaluation
History and Physical Examination
The physical examination should assess mental status and confirm the level of the neurologic deficit (if present). Perineal sensation and pelvic floor muscle tone are particularly important. Reflexes are also important, but the bulbocavernous, cremasteric, and anal reflexes are sometimes difficult to elicit, even in neurologically intact patients. The skin of the perineum and the state of bladder support should be assessed. In women, a standard pelvic examination is warranted to assess for adequate estrogenization and any evidence of vaginal wall support defects. Good estrogenization is evidenced by a pink and moist vaginal mucosa with good rugae, whereas lack of estrogenization reveals a pale, dry, and smooth vaginal mucosa. Evidence of cystocele or pelvic organ prolapse can be seen in vaginal wall support defects. The prostate in males should be evaluated, but prostate size or consistency alone is not a good indicator of obstruction. It is also important to assess the patient’s motivation, lifestyle, body habitus, and other physical impairments including an assessment of upper limb function, lower limb function, and spine range of motion.
Diagnostic Tests
Indications
The bladder findings on urodynamic studies cannot be used alone to determine the level of neurologic lesion. For example, a suprasacral neurogenic bladder from a complete spinal cord injury can remain areflexic, and a conal or cauda equinal bladder can exhibit high pressures from poor compliance (Table 28-3). Although the anatomic level of the neurologic lesion can suggest to the clinician the most likely pattern of bladder dysfunction, urodynamic testing should be performed to confirm this. Functional bladder studies in traumatic spinal cord injury are best deferred until spinal shock has cleared.
Table 28-3 Urodynamic Definitions
| Term | Definition |
|---|---|
| Bladder | |
| Hyperactivity | Contractions of the detrusor during filling |
| Hypocontractility | Unsustained contractions causing failure to empty |
| Areflexia | Absent contractions with attempt to void |
| Compliance | Change in volume divided by change in baseline pressure with filling (<10 mL/cm H2O abnormal; 10 to 20 mL/cm H2O borderline if capacity reduced) |
| Outlet | |
| Detrusor–sphincter dyssynergia | |
| 1. At bladder neck | Usually in high tetraplegic patients with autonomic hyperactivity |
| 2. At striated sphincter | Uncoordinated pelvic floor and striated sphincter contraction with detrusor contraction during attempts to void |
| Nonrelaxing sphincter | Poor voluntary relaxation of voluntary sphincter in patients with areflexia attempting to void by the Valsalva maneuver |
| Decreased outlet resistance | Incontinence caused by damage to the bladder neck or striated sphincter, pelvic floor descent, or denervation |
Upper Tract Tests
Lower Tract Tests
Urinalysis, Culture, and Sensitivity Testing
These tests are done routinely for all patients with neurogenic bladder disease and should be repeated as often as necessary or at the very least at routine follow-up annually. These would also be recommended before invasive procedures in cases of suspected UTI (i.e., with cloudy, foul smelling, or bloody urine) or with new lower urinary tract symptoms such as incontinence, frequency, etc. Bacteriuria should be treated before any invasive urologic procedure or test is performed.
Postvoid Residual
By itself, a low postvoid residual (PVR) of less than 20% of capacity is not indicative of a “balanced” bladder as it was once defined because high intravesical pressures can be present despite low PVR values. The PVR is simple to determine and clinically useful, especially when compared with previous recordings and considered in conjunction with the bladder pressure, clinical symptoms, and the appearance of the bladder wall. PVRs can vary throughout the day. A catheter insertion has been used for PVR in the past, but there are now simple US machines that noninvasively obtain the PVR.9
Cystometrography
Water CMGs are best obtained with a two-channel catheter, with one channel used for filling and the other for pressure recording. A rectal pressure trace is also helpful in many patients to help distinguish intravesical pressure variations (resulting from intraabdominal transmission) from contractions of the detrusor itself. Reported filling rates vary but usually range from 25 to 60 mL/min. During filling, patients are asked to suppress voiding. Normal values include a capacity of 300 to 600 mL, with an initial sensation of filling at approximately 50% of capacity. The sensation of normal fullness is said to be appreciated in the lower abdomen with a sense of urgency in the perineum. The change in volume divided by the increase in baseline pressure during filling (i.e., in the absence of a detrusor contraction) describes the bladder’s compliance. This value should be greater than 10 mL/cm H2O, and 10 to 20 mL/cm H2O is considered borderline if the bladder capacity is reduced. The patient is asked to suppress detrusor contractions during this test. Any detrusor contraction during the test, usually defined as a phasic pressure change of more than 15 cm H2O, is abnormal. If the patient is neurologically intact, these contractions are referred to as uninhibited. If the patient has a suprasacral or supraspinal lesion, these contractions are called hyperreflexic.2 Overactive or hyperactive are terms now used to describe both types of contractions.
Sphincter Electromyography
Sphincter electromyography (EMG) can be combined with the CMG or preferably with a full multichannel videourodynamic study.28 Recordings have been made with a variety of electrodes (monopolar, coaxial needle, and surface electrodes) from the levator, perianal, or periurethral muscles. Because some authors claim there is a functional dissociation between these muscle groups, periurethral recordings are preferred. The integrated EMG is displayed on the same trace as the bladder pressure. EMG activity gradually increases as bladder capacity is reached during bladder filling, and then becomes silent just before voiding. Low levels of EMG activity with no recruitment during filling are a common pattern in complete spinal cord injury. When a reflex detrusor contraction occurs in these patients, the EMG activity in the sphincter can increase rather than decrease. With this detrusor–sphincter dyssynergia, voiding often occurs toward the end of the detrusor contraction because the striated sphincter relaxes more quickly than the smooth muscle of the bladder. This type of sphincter EMG does not display individual motor units and cannot be used for the evaluation of infrasacral denervation of the pelvic floor musculature (for which standard needle EMG is needed). Diagnostic integrated EMG recordings from the external urethral sphincter are difficult to obtain and are invasive and painful in patients who are sensate. The fluoroscopic appearance of the urethra is an alternative method of determining sphincter dysfunction and is preferred after distal sphincterotomy if recurrent distal sphincter dyssynergia is suspected (Figure 28-6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree