Management of Acute and Chronic Tendon Injury
Ryan H. Fitzgerald
Despite being less common than osseous or ligamentous trauma, tendon injuries in the foot and ankle present a significant management challenge to the physician and can demonstrate long-term functional consequences if not addressed correctly. Untreated tendon injuries can lead to reduced function, prolonged disability, as well as joint destabilization and subsequent arthrosis. To prevent these sequelae, it is important that the surgeon understand the various nuances regarding the management of acute and chronic tendon injuries to help establish the most rapid return to function for the patient.
The mechanism of action for tendon trauma can be seen to be either endogenous or exogenous tendon trauma. Endogenous tendon trauma results from excessive intensity movement that creates inordinate tension within the tendon; normal-intensity movement performed in a nonphysiologic condition, as in high-level sports activity; or lower-intensity movement in a previously diseased tendon. Conversely, exogenous tendon trauma is due to lacerations or direct contusion of the tendons. Regardless of the mechanism of action, the principles guiding the evaluation and management of tendon trauma remain the same.
EVALUATION
Acute tendon injuries are most commonly diagnosed through a combination of thorough history and clinical examination. When evaluating a patient who has suffered lower extremity trauma, it is necessary to have a high clinical suspicion for tendon injury because even minor trauma may result in significant tendon trauma. While imaging studies are useful in confirming the diagnosis of tendon injury and establishing the degree of damage, it is important to recognize that the preliminary diagnosis of tendon injury is largely made through thorough examination of the muscle function of the affected extremity.
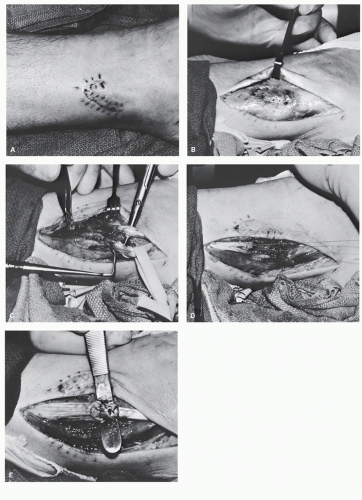
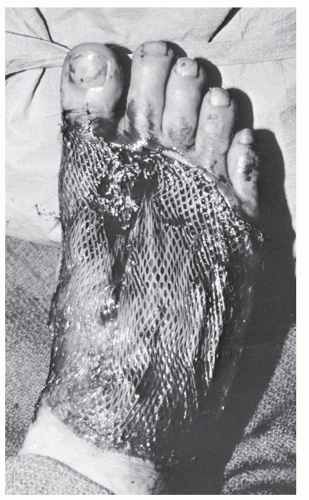
In those instances in which a patient is undergoing débridement of lacerations, it is important to evaluate the appearance and competence of tendons, even in those cases in which it seems unlikely that tendon trauma will have occurred, such as digital lacerations or puncture wounds. Unfortunately, superficial tendon lacerations as a consequence of this sort of trauma are frequently missed or neglected in the emergency room (Fig. 100.1), and this situation can lead to increased patient morbidity, increased need for complicated reconstructive efforts when the diagnosis is ultimately made, and overall reduced function.
IMAGING TECHNIQUES
RADIOGRAPHS
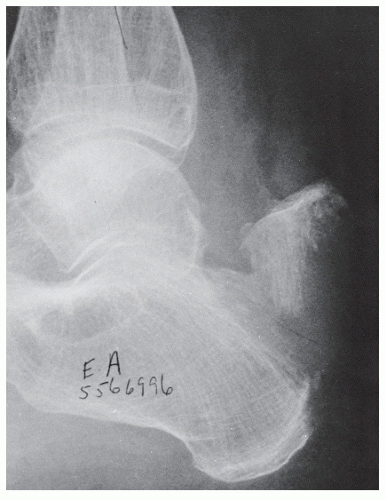
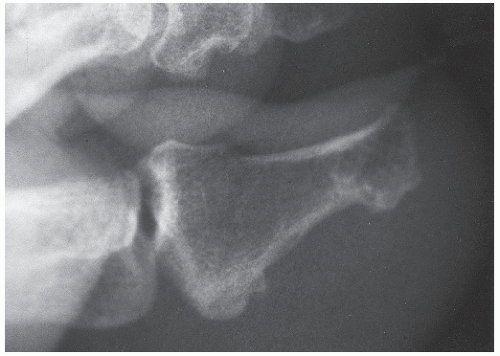
Radiographs assist in the diagnosis of tendon trauma because they help rule out any concomitant or occult fractures, avulsion of the tendinous insertion, or injuries to sesamoid bones within tendons (Fig. 100.2). Furthermore, radiographs are useful in the evaluation of fractures and dislocations that may entrap tendons, and, in the case of Achilles tendon pathology, are helpful in evaluating Kager triangle.
COMPUTED TOMOGRAPHY SCANS
Computed tomography (CT) is an imaging modality that has a significant role in the evaluation of trauma to the foot and ankle. With CT scans, one can visualize the osseous and soft tissue relationships in the foot and ankle and can observe any derangements that might exist. On CT scan, tendons appear as homogenous, well-circumscribed, round or oval densities surrounded and highlighted by fat (1). On CT, tendons appear to have higher attenuation values than surrounding muscle and can therefore be distinguished between them; the musculotendinous junction appears as a round area with increasing density at the periphery (2,3). Because CT scans utilize radiation to create images, CT is useful in visualizing tendon trauma associated with significantly solid objects, such as calcifications or bone fragments that can complicate tendon dislocation and rupture (4). When evaluating tendon pathology utilizing CT scans, a complete tendon rupture can be visualized by an absence of a portion of the tendon, with the void filled by either fat or fluid (1). Partial ruptures can be visualized by observing an increase in tendon girth and radiolucent areas within the tendon substance. While tendon pathology can be imaged utilizing CT to some degree, the overall evaluation of tendon trauma via CT is limited due to the inability to visualize the tendons in the coronal and sagittal planes, and therefore utilization of other imaging modalities is recommended for the evaluation of tendon abnormalities unless the physician is seeking to evaluate the patient for potential for concomitant osseous injury.
TENOGRAPHY
Tenography involves the indirect visualization of tendons within their sheaths using plain film radiography and the injection of a radiopaque dye and can be useful in determining frank ruptures, partial tears, and other tendon lesions. Unfortunately,
this procedure is invasive and can prove to be technically difficult. There can be a high number of false positives if the dye is injected along the outside of the tendon sheath, which can yield an image that can be misinterpreted as a rupture. Furthermore, because tenography yields indirect visualization of the tendon, it may be difficult to detect partial ruptures and longitudinal splits, especially when there is swelling that can obscure anatomic landmarks or in instances in which previous injury has caused scarring to occlude the tendon sheath (4).
this procedure is invasive and can prove to be technically difficult. There can be a high number of false positives if the dye is injected along the outside of the tendon sheath, which can yield an image that can be misinterpreted as a rupture. Furthermore, because tenography yields indirect visualization of the tendon, it may be difficult to detect partial ruptures and longitudinal splits, especially when there is swelling that can obscure anatomic landmarks or in instances in which previous injury has caused scarring to occlude the tendon sheath (4).
ULTRASONOGRAPHY
In recent years, with the advent of higher resolution ultrasound, ultrasonography has become more utilized in the evaluation of tendon trauma. Ultrasonography is useful in evaluation of tendon trauma because it provides a quick, inexpensive, nonradiating, and noninvasive imaging modality that can be utilized to evaluate for tendon pathology (5,6). This imaging modality produces images based upon the sound reflectivity of the object being imaged and the acoustic impedance that the object demonstrates, and unlike magnetic resonance imaging (7), ultrasound images are not degraded by the presence of orthopaedic implants, and can be used in patients with cardiac pacemakers without risk.
Ultrasonography has been historically useful in examination of Achilles tendon trauma, but as the technology has advanced, and resolution increased, practitioners have begun to evaluate all types of soft tissue trauma (8,9,10,11,12,13 and 14). Despite increased usage in the evaluation of lower extremity trauma, it must be remembered that, due to its physical properties, ultrasonography is less useful in visualizing deeper soft tissue and osseous structures (15).
In the evaluation of tendon trauma, real-time ultrasound machines are preferred over static B-mode systems because these real-time machines allow for quicker identification of the involved tendons as well as dynamic examination of tendon function during contraction and relaxation of the muscle. With regard to transducers, linear array transducers are preferred over sector probes because their high-frequency beams are perpendicular to the superficial structures (16). Often, a standoff pad will be utilized to improve the interface between the subcutaneous tissues, skin, and the probe. Use of a standoff pad also places the tendon in the optimal focal zone to allow for increased visualization and evaluation (17). When evaluating tendons in the lower extremity, most often a very high-frequency probe is utilized (7.5, 10, 15, or 20 MHz) due the fact that most tendons and soft tissue structures of the foot and ankle are generally superficial in location. High-frequency probes, which have a short depth of field (3 to 4 cm), give excellent spatial and contrast resolution (18,19).
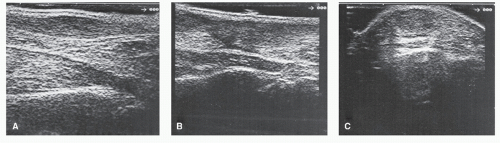
When evaluating tendinous lesions, a combination of longitudinal and transverse scans provide the best three-dimensional localization for visualization of potential defects (Fig. 100.3). The fibrillar texture of tendons and their inherent echogenic profile aid in the distinction of these structures from surrounding fatty tissue, which is more echogenic. It is important for the physician to recognize, however, that echogenicity of tendons is dependent somewhat on the angle at which the transducer is applied (20). This quality is known as anisotropy. When scanned perpendicular to its longitudinal axis with a linear array transducer, a tendon can be seen to be significantly
more echogenic than muscular structures; however, should the angle be reduced, the echogenicity of the tendon can be seen to decrease relative to that of muscle, making it difficult to distinguish between the two (20). Muscle tissue does not demonstrate anisotropy, and therefore has a relatively constant echogenic profile, which, when the transducer is held perpendicular, is easily distinguished from than of tendons (21,22 and 23). Thus, it is necessary that the transducer be applied in perpendicular fashion relative to the tendon when seeking to evaluate tendon trauma.
more echogenic than muscular structures; however, should the angle be reduced, the echogenicity of the tendon can be seen to decrease relative to that of muscle, making it difficult to distinguish between the two (20). Muscle tissue does not demonstrate anisotropy, and therefore has a relatively constant echogenic profile, which, when the transducer is held perpendicular, is easily distinguished from than of tendons (21,22 and 23). Thus, it is necessary that the transducer be applied in perpendicular fashion relative to the tendon when seeking to evaluate tendon trauma.
When evaluating tendon trauma via ultrasonography, the physician must understand what is being visualized. Tendon tears, either partial or total, are represented by discontinuity of fibers and the presence of hematoma. Fresh clot is highly echogenic initially when imaged at frequencies of 5.0 to 10.0 MHz, but this disappears within 96 hours due to hemolysis (24). Swollen portions of tendons, usually near the rupture site, can also be observed. However, false-negative sonogram results can occur because of the similar echogenic properties of tendon and hematoma. This is especially true in neglected ruptures or late examinations when the hematoma is organizing (25). False-positive results may be obtained owing to the hypoechogenicity that results when the tendon lies oblique to the ultrasound plane in longitudinal scanning or when the tendon is not strictly perpendicular to the ultrasound plane in transverse scanning. This may also occur when deviations or disruptions of the tendon are not reevaluated in another plane (15).
As previously discussed, ultrasonography has become a useful modality in the evaluation of soft tissue trauma in the foot and ankle. Recent research has demonstrated that, when compared with surgical findings, ultrasonographic evaluation was able to identify all tendon tears correctly in a variety of structures at the ankle in 54 patients (26). In fact, in four patients, the ultrasonographic evaluation identified tendon pathology that was not recognized at the time of surgery. Some researchers have believed that ultrasonography provided better contrast resolution for tendons at the foot and ankle level than that of MRI (26). In these studies, however, the authors note that the major limitation was the fact that the accuracy of the study was highly dependent on the individual performing the evaluation.
The ability to evaluate tendons in real time provides the practitioner greater diagnostic ability when determining tears, complete ruptures, and intratendinous changes (27). This is especially so with the increasing numbers of practitioners who have ultrasound machines in their offices. Furthermore, ultrasonography also provides the additional benefit of rapid hard-copy documentation, which is significant in today’s litigious society. The greatest single hindrance and limitation associated with the use of ultrasonography in the evaluation of tendon pathology is that the quality of the study is significantly user dependent. Lack of familiarity with the equipment or with musculoskeletal ultrasound imaging and interpretation can limit the diagnostic accuracy of the study (16). For this reason, ultrasonography remains a less popular imaging modality as compared with MRI or CT for the evaluation of tendon abnormalities.
MAGNETIC RESONANCE IMAGING
Perhaps the most useful imaging modality in the evaluation of tendon pathology and soft tissue visualization is MRI. MRI allows the practitioner to visualize tissues directly, allowing the physician to evaluate for partial and complete tendon tears, tendonitis, and tenosynovitis, and this modality can be utilized to confirm the diagnosis of subtle tendon pathologies that can be difficult to detect via other modalities such as internal derangements of the tendons. MRI demonstrates significant advantages over other imaging modalities because it allows for multiplanar imaging, provides excellent contrast resolution, is noninvasive, and does not utilize ionizing radiation to produce images (28). Instead, MRI utilizes magnetic fields that are passed across the patient to generate the appropriate images (28,29 and 30).
When evaluating for tendon pathology via MRI, it is important to note any changes in signal density along the course of the tendon or within the substance of the tendon itself. These changes such as thickening, thinning, or contour irregularities are findings consistent in abnormal tendons (31). Additionally, fluid collections can be visualized, which in some cases may also suggest abnormality; however, it must be remembered that small fluid collections along tendon sheaths of muscles, such as in the flexor hallucis longus, can be a normal physiologic finding (32). When discussing the evaluation of tendons via MRI, it is also important to address the concept of the “magic angle effect,” which is a phenomenon that occurs when a tendon is evaluated utilizing a spin-echo sequence and the tendon is oriented 55 degrees from the main magnetic field. This “magic angle effect” creates the illusion of a tendon tear in an otherwise normal tendon and can lead one to a false-positive (33,34). Despite this, MRI has become one of the mainstays for evaluation of tendon pathology, especially as it has become more widely available to the medical community (18,29,35,36).
GENERAL PRINCIPLES OF TENDON INJURY
When discussing tendon pathology, it is important to understand the mechanism of injury to truly understand the nature and function of any repair that may follow. Studies have demonstrated that the most common cause for tendon rupture— either partial or complete—is eccentric overloading of the muscle-tendon unit (37,38 and 39). This eccentric loading is by no means, however, the only common mechanism, and it should be noted that often patients present with tendon injuries following a direct blow by the sharp edge of an object along the course of a physiologically taut tendon or with tendon injuries sustained following open traumatic lacerations.
TENDON RUPTURE
The tendon is the strongest component of the musculotendinous unit, and true ruptures of normal, healthy tendon are rare (40). Research has demonstrated that a healthy, intact tendon does not rupture when it is subjected to severe strain alone— muscle strain being defined as indirect injury to muscle via a tension overload in the passive muscle or eccentric overload in the actively contracting muscle (40,41). Indeed, animal models have demonstrated that greater than 50% of tendon fibers must be severed to create a tendon rupture in the context of significant strain (42). Furthermore, research has demonstrated that normal, healthy tendon, in the absence of strain, does not rupture even in instances in which nearly 75% of tendon fibers had been severed (38,43). In healthy tendon, injuries such as osseous avulsion or avulsion through the muscle belly are far
more likely to occur with significant muscle strain than intrasubstance tendon rupture (44).
more likely to occur with significant muscle strain than intrasubstance tendon rupture (44).
TABLE 100.1 Location of Ruptures of the Lower Leg | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
When considering the demographics of patients who present with tendon ruptures, it is important to recognize that such ruptures are more commonly associated with middle-aged and elderly patients (45). Studies have demonstrated that tendon ruptures are approximately six times more commonly observed in the upper extremity than the lower extremity, and generally speaking, these types of injuries are more commonly observed in men rather than women (Table 100.1) (46,47).
When tendon ruptures are observed, they are commonly associated with diseased tendon, especially in the context of tendons that have been stressed to failure by increased muscle strain. While exact measurement is difficult, several authors have attempted to classify the degree of tendon injury sustained quantitatively with the level of muscle strain applied to the musculotendinous unit (48). In this classification system, a first-degree strain yields less than 5% disruption of the tendinous structure. Second-degree strain demonstrated increased disruption of the tendon; however, there is incomplete rupture. Third-degree strain represents complete rupture with potential discontinuity of the tendon and major loss of function (40,49). It is important, when involved in the management of these injuries, to recognize that a patient’s failure to regain full flexibility and normal strength of the musculotendinous unit following muscle strain places that patient at increased risk of further injury.
In addition to muscle strain, there are a number of systemic and local processes that can predispose a patient to tendon rupture. Spontaneous ruptures have been reported in patients who have associated systemic and local disease processes that can weaken a tendon to failure. Medical conditions such as lupus erythematosus, diabetes mellitus, rheumatoid arthritis, collagen vascular diseases, and hyperparathyroid due to dialysis have been shown to weaken tendons such that spontaneous tendon rupture can occur (50,51,52,53 and 54). Additionally, biomechanical abnormalities, decreased local blood supply, systemic steroid use, gout, and fluoroquinolone therapy may predispose the tendon to rupture. In these cases, the disease process creates chronic inflammation and leads to continued tendon degradation and will eventually lead to rupture if the weakened tendon is stressed sufficiently.
Localized inflammatory processes can also lead to tendon weakness and rupture. Paratendinitis is an inflammatory process that occurs in the paratenon surrounding tendons that possess no tendon sheath. Conversely, tenosynovitis is an inflammatory process that occurs around tendons containing a tendon sheath (55). Peritendinitis is a catch-all term, which has been used to denote either paratendinitis or tenosynovitis (4). Peritendinitis, when it occurs in the lower extremity, is usually due to overuse or biomechanical abnormality with associated compensation (40,56). Commonly, the flexor hallucis longus, posterior tibialis, and Achilles tendon are involved. When defining degenerative lesions with the tendon, the term tendinosis is used, and it should be noted that rupture can occur in the context of peritendinitis with tendinosis, or with tendinosis alone (57). When the disease process is mild, the tendon may show only microscopic changes; however, as inflammation persists, studies have demonstrated increased thickening of the peritendinous sheath with enlargement and fraying of the tendon. If inflammation persists, the tendon will continue to degenerate and frank rupture will occur. Several authors have hypothesized that repetitive mechanical stress results in disruption of the collagen structure of the tendon and that subsequent chronic inflammatory changes permanently alter the capacity for collagen synthesis, thus yielding decreased healing potential (4,52). In this way, recurrent microtrauma with incomplete healing and persistent inflammation predisposes a tendon to continued attenuation and eventual rupture. This is especially true in the watershed areas that demonstrate reduced vascularity
LACERATION
Tendon lacerations are commonly observed in patients suffering lower extremity trauma, and therefore it is important that motion and strength of tendons be addressed as part of the thorough physical exam (58). Surgical repair of acute tendon pathology is usually employed for open lacerations, especially in children (59,60). Often, open repair of tendons includes débridement of nonviable tendon elements and anatomic restoration of the tendon with either primary or delayed primary closure (61). Additionally, patients suffering acute tendon laceration following lower extremity trauma often require aggressive wound care, appropriate prophylactic antibiotic therapy, tetanus prophylaxis, and reconstruction or appropriate closure of open wounds (62).
In the context of tendon lacerations, it is important to note the time since the injury. Following débridement of all nonviable tissue, one must determine whether primary repair or delayed repair is indicated. Primary repair is indicated in those instances when such repair is initiated 6 to 8 hours following the injury. In those instances in which a longer interval of time has occurred since the initial injury, débridement with secondary tendon repair is indicated. In all cases, if there is any doubt regarding the viability of the tissue or the level of contamination of the wound, it is best to perform serial débridement and delay tendon repair.
TENDON REPAIR
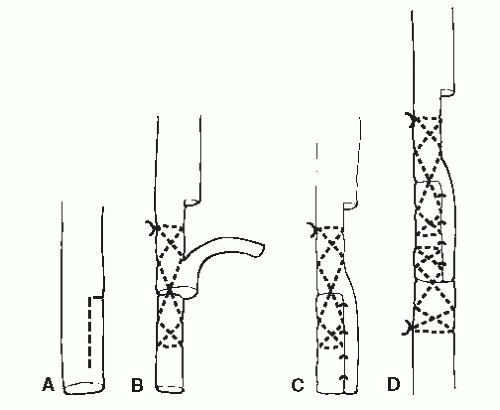
Regardless of the mechanism of injury, the goal in tendon repair remains the same: a union of adequate tensile strength that maintains gliding function as quickly as possible. It is vital that the surgeon limit further surgical trauma to the area of injury, and therefore, atraumatic technique is primary when
considering operative repair of an injured tendon. Often, tendons are treated by a combination of surgical repair and postoperative immobilization. In these instances, suture selection and application as part of the primary surgical repair is vital. Studies have demonstrated that for the first 10 days following surgery, the tensile strength of the repaired tendon relies solely on the suture material and the technique of its application during the repair (8,47,63). Ideally, the suture technique should appose the free tendon ends while allowing some early passive motion without generating any gapping. At this point in the repair, tissue handling becomes important as well because it is necessary that the suture should not strangle the tissue or otherwise impede the blood supply to the healing tendon (53). Encircling the tendon and strangulation of the ends can prolong the healing process and potentially lead to tissue necrosis. To establish adequate end-to-end repair, it is necessary that each end of the tendon contain viable tissue.
considering operative repair of an injured tendon. Often, tendons are treated by a combination of surgical repair and postoperative immobilization. In these instances, suture selection and application as part of the primary surgical repair is vital. Studies have demonstrated that for the first 10 days following surgery, the tensile strength of the repaired tendon relies solely on the suture material and the technique of its application during the repair (8,47,63). Ideally, the suture technique should appose the free tendon ends while allowing some early passive motion without generating any gapping. At this point in the repair, tissue handling becomes important as well because it is necessary that the suture should not strangle the tissue or otherwise impede the blood supply to the healing tendon (53). Encircling the tendon and strangulation of the ends can prolong the healing process and potentially lead to tissue necrosis. To establish adequate end-to-end repair, it is necessary that each end of the tendon contain viable tissue.
When performing primary repair of tendons, most commonly nonabsorbable sutures are used to maximize the strength of the repair; this is especially true when repairing larger tendons with anticipation of higher tensile loading. The use of absorbable sutures has also been described in primary tendon repair, particularly in situations in which individual segments of the flexor digitorum longus or extensor digitorum longus tendons have been compromised and are being repaired (28,57). In these instances, absorbable sutures have been shown to maintain adequate strength for a sufficient period of time to support tendon healing (64,65). Furthermore, absorbable sutures are often utilized to provide addition support in conjunction with nonabsorbable sutures in primary tendon repairs.
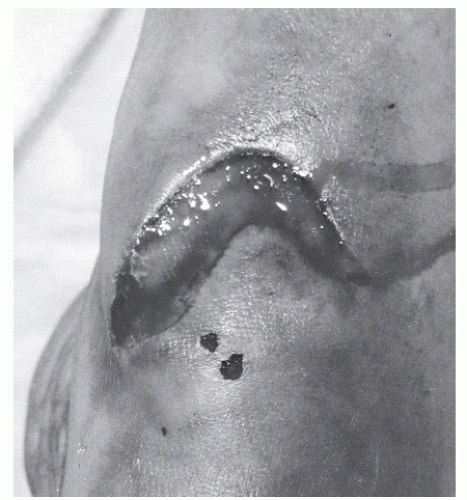
Patients who present with crush injuries that result from a blunt or oblique trauma often generate a greater degree of soft tissue damage (Fig. 100.4), and often the level of damage may not be readily apparent upon initial presentation (61). Tissue necrosis in the zone of injury may not become evident for several days, and in these instances, multiple débridement may be necessary to fully remove all necrotic tissue (11). In these cases, a secondary repair of the tendon is required; often, this occurs in conjunction with other procedures such as skin grafting to achieve final wound closure. Such procedures are discussed in detail elsewhere in this textbook. In patients undergoing secondary repair following tendon injury, it is essential that early soft tissue coverage be provided to the tendon to reduce the risk of adhesions (Fig. 100.5) (66).
Occasionally, there are circumstances in which, following traumatic injury or débridement, the injured tendon ends do not have sufficient length to provide for direct apposition for repair. In these instances, there are multiple techniques available to the surgeon to provide the necessary length to provide for necessary function. These include V-Y lengthening, turndown onlay grafting, or the use of autogenous or cadaveric graft tissue to span the gap between proximal and distal tendon stumps (57,67). When selecting a graft to fill the tendon defect, autograft is preferred, and when necessary, the plantaris, peroneus tertius, or peroneus can be harvested (19). If a shorter graft is required, often segments of the extensor digitorum longus, extensor digitorum brevis, or the extensor hallucis brevis are utilized. As a general principle, when selecting an autograft, one
should make every effort to match the diameter of the autograft with the recipient tendon—doing so reduces adhesions as the tendons heal (68,69). As previously discussed, atraumatic surgical technique is of extreme importance to reduce the risk of tissue necrosis and to maintain viable tendon margins. When utilizing grafted tendon to fill a defect, the transplanted tendon is initially avascular and serves simply to provide a fibrotic strut to bridge the gap between viable tendon ends (70,71). As the tendon heals, the transplanted tendon is eventually incorporated into the recipient tendon. By the end of the 3rd week, the tendon graft will be strong enough for gentle mobilization; by the end of 10 to 12 weeks, the graft will have become completely incorporated to the host tendon (66,69,71).
should make every effort to match the diameter of the autograft with the recipient tendon—doing so reduces adhesions as the tendons heal (68,69). As previously discussed, atraumatic surgical technique is of extreme importance to reduce the risk of tissue necrosis and to maintain viable tendon margins. When utilizing grafted tendon to fill a defect, the transplanted tendon is initially avascular and serves simply to provide a fibrotic strut to bridge the gap between viable tendon ends (70,71). As the tendon heals, the transplanted tendon is eventually incorporated into the recipient tendon. By the end of the 3rd week, the tendon graft will be strong enough for gentle mobilization; by the end of 10 to 12 weeks, the graft will have become completely incorporated to the host tendon (66,69,71).
TENDON HEALING
Following injury, the initial healing of an injured tendon occurs over a 4-week period. It is important that the physician understand the stages of tendon healing when treating patients who have sustained a tendon injury. During the 1st week, the severed ends of the tendon become loosely joined by granulation tissue (71). Throughout the 2nd week, paratenon vascularity increases, and during the 3rd week, collagen fibrils align longitudinally, which provides the healing tendon moderate tensile strength, and by the 4th week, edema has sufficiency diminished to allow guarded limited range of motion to begin (72). At 4 weeks, the patient can be progressed from immobilization to a gradual return to full function as the tendon continues to heal (73,74). Return to function is of extreme importance because continued immobilization increases the risk of tissue adhesions along the healing tendon as well as the risk of other comorbidities, such as deep vein thrombosis, which is a significant concern in patients who have been immobilized or have recently undergone surgery (74).
EXTENSOR TENDONS
On the dorsum of the foot, the tibialis anterior, extensor hallucis longus, extensor digitorum longus, and peroneus tertius pass deep to the fibro-osseous canal known as the extensor retinaculum. The structure is composed of two bands—the superior and inferior retinacula—and serves to stabilize the extensor tendons and maintain mechanical advantage as the tendons cross the ankle joint toward their insertions. Injuries to the extensor tendons about the dorsum of the foot and ankle often are due to traumatic laceration (51,75). It is important that lacerations to the dorsum of the foot and ankle be thoroughly evaluated for potential underlying tendon injury because often these lacerations appear benign (58,73).
TIBIALIS ANTERIOR
The tibialis anterior is a strong dorsiflexor of the ankle and inverter of the subtalar and midtarsal joints. It becomes tendinous at the level of the distal tibial metaphysis just before becoming enclosed in a synovial sheath. The tendon passes under the superior and inferior retinacula and inserts along the medial aspect of the medial cuneiform and first metatarsal base.
Disruption of the tibialis anterior tendon is most commonly associated with open wound or laceration (76). Closed ruptures of the tibialis anterior tendon are uncommon (77). This is likely due to the fact that the tibialis anterior muscle functions primarily during the swing phase of gait and therefore functions in a predominately unloaded state (78,79 and 80). As with other tendon ruptures, ruptures of the tibialis anterior more commonly present in male patients over the age of 45 (76,77,81). Closed ruptures most often occur following minimal trauma in which unexpected plantarflexion eccentrically stresses a contracting tibialis anterior muscle. Commonly, this mechanism of injury is observed in soccer and tennis players (82). Additionally, rupture of the tibialis anterior has been shown to have an association with chronic rupture of the posterior tibial tendon. Rupture of the tendon following steroid injection has also been demonstrated by a multitude of studies.
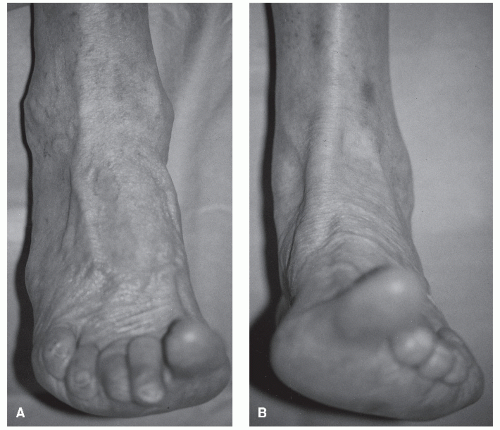
Patients suffering from tibialis anterior tendon rupture generally present reporting a weakness of the foot or ankle with associated swelling along the anteriomedial aspect of the foot and ankle (77,78). Additionally, the patient may complain of a snapping sensation in the upper part of the arch and a sharp pain. On rare occasions, there may be a palpable gap in the tendon at the site of rupture (Fig. 100.6). Ruptures of the anterior tibial tendon most commonly occur 1 to 2 cm proximal to the insertion along the medial aspect of the medial cuneiform (83). This area has been demonstrated to have a limited blood supply. Disruption may also occur at the myotendinous junction, and several studies have demonstrated increased risk of tenosynovitis and subsequent rupture in the tibialis anterior tendon at the level of the inferior edge of the retinaculum due to the potential for abrasion as the tendon traverses the tunnel (84). Often, there is retraction of the proximal stump, creating a bulbous enlargement above the anterior medial ankle.
The pain and weakness appear to be maximal within the first few hours of injury, but pain, swelling, and ecchymosis often resolve quickly and therefore may not be notable if there was delay in patient presentation (76). Active dorsiflexion of the ankle is reduced in patients suffering tibialis anterior tendon rupture as compared with the contralateral side, and in these patients, dorsiflexion is accomplished via extensor hallucis and digitorum longus tendons. Clawing of the lesser digits may be observed due to the extensor substitution phenomenon (85). With dorsiflexion, eversion of the foot may be noted due to the unopposed action of the peroneal musculature (86). On examination of gait, the patient may be noted to walk with the heel everted during swing phase and with a mild slap of the foot upon heal contact. Additionally, the patient will demonstrate difficulty walking on heels. The patient may compensate for the loss of dorsiflexion during swing phase by adapting to a mild steppage gait (87). Often, these patients’ symptoms mimic radiculopathy with dropfoot, and a thorough neuromuscular exam should be performed to confirm the presence of active function in the other muscles innervated by the deep and superficial branches of the peroneal nerve to rule out the possibility of a peripheral neuropathy (76).
Electromyography studies can confirm the absence of motor neuropathy, and ultrasound can be utilized to confirm the diagnosis of tendon rupture (78,79,88). Ruptures of the tibialis anterior may also be observed on CT, but these injuries are best visualized, as discussed previously, on sagittal plane MRI (Fig. 100.7) (89). On MRI, the tendon will appear discontinuous, with likely increased fluid signal noted along the distal course of the tendon sheath.
Conservative therapy is indicated in elderly patients and those that are considered less active. Additionally, in those injuries that involve avulsion of the tibialis anterior insertion in which displacement of the tendon is less than 5 mm,
conservative therapy can be indicated (90). In the management of tibialis anterior tendon ruptures, conservative therapy consists of below-knee, non-weight-bearing cast immobilization with the foot in a dorsiflexed and inverted position for approximately 4 to 6 weeks (73). In these patients, the tendon often becomes adhered more proximally so there is often an overall decrease in dorsiflexion and strength noted in subsequent physical exams. For these patients, an ankle-foot orthosis may be indicated if loss of dorsiflexion is significant (91).
conservative therapy can be indicated (90). In the management of tibialis anterior tendon ruptures, conservative therapy consists of below-knee, non-weight-bearing cast immobilization with the foot in a dorsiflexed and inverted position for approximately 4 to 6 weeks (73). In these patients, the tendon often becomes adhered more proximally so there is often an overall decrease in dorsiflexion and strength noted in subsequent physical exams. For these patients, an ankle-foot orthosis may be indicated if loss of dorsiflexion is significant (91).
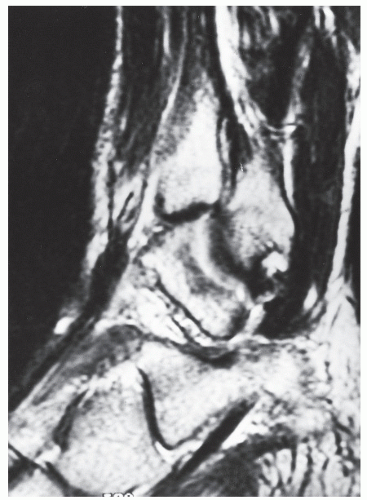 Figure 100.7 Magnetic resonance imaging of a ruptured tibialis anterior tendon demonstrating the disrupted tendon ends surrounded by fluid signal. |
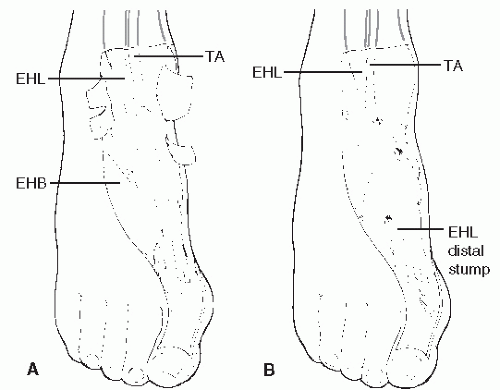
For the majority of patients, surgical repair is indicated. This is especially true for those patients who are younger or middle aged and in those patients who wish to maintain a higher level of physical activity. In those cases in which the injury is relatively recent, and when no significant retraction is present, end-toend repair can be performed (43). In those cases in which the tendon has avulsed from the insertion at the medial cuneiform and there has been limited retraction of the proximal portion of the tendon, the tendon can be advanced and tenodesed to the medial cuneiform by way of a drill hole or tendon anchor. If a significant gap exists between tendon ends such that primary tendon repair cannot be accomplished, a tendon graft may be employed. Several grafts have been described in the literature, including free tendon graft from the extensor digitorum longus, a tendon lengthening utilizing a split portion of the proximal, intact tibialis anterior tendon (89,94), or a free peroneus brevis graft (Fig. 100.8) (76,92). When harvesting peroneus brevis as a free graft, the proximal and distal stumps of the remaining peroneus brevis tendon are sutured to the peroneus longus tendon (93). Another technique that has been described in the literature utilizes the extensor hallucis longus. In this procedure,
the proximal stump of the tibialis anterior tendon is sutured to the adjacent extensor hallucis longus, which is severed at the metatarsophalangeal joint. The extensor hallucis longus is then rerouted to the insertion of the tibialis anterior tendon (Fig. 100.9) (94). Reattachment of the extensor hallucis longus-proximal tibialis anterior complex can be accomplished through a drill hole in the medial cuneiform by suturing of the tendon back onto itself, by a suture passed through the sole of the foot and tied over a button, or by means of bone anchors, screw and washer fixation, or direct anastomosis to the anterior tibial tendon stump at the cuneiform (93,94 and 95). The distal aspect of the remaining extensor hallucis longus can then be anastomosed to the extensor digitorum longus tendon to the second digit or sutured to the tendon of the extensor hallucis brevis (93).
the proximal stump of the tibialis anterior tendon is sutured to the adjacent extensor hallucis longus, which is severed at the metatarsophalangeal joint. The extensor hallucis longus is then rerouted to the insertion of the tibialis anterior tendon (Fig. 100.9) (94). Reattachment of the extensor hallucis longus-proximal tibialis anterior complex can be accomplished through a drill hole in the medial cuneiform by suturing of the tendon back onto itself, by a suture passed through the sole of the foot and tied over a button, or by means of bone anchors, screw and washer fixation, or direct anastomosis to the anterior tibial tendon stump at the cuneiform (93,94 and 95). The distal aspect of the remaining extensor hallucis longus can then be anastomosed to the extensor digitorum longus tendon to the second digit or sutured to the tendon of the extensor hallucis brevis (93).
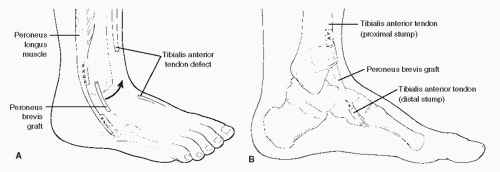 Figure 100.8 Peroneus brevis tendon graft used to repair a defect in the tibialis anterior tendon. Anterolateral (A) and medial (B) views of the procedure. |
Following open repair, short-leg cast immobilization is typically preferred, with non-weight-bearing from approximately 4 to 6 weeks. If grafting techniques have been employed, then the patient is generally casted for a full 6 weeks. Once the case is removed, the patient may be transitioned to a CAM walker or other protective device for a variable period while physical therapy for range-of-motion and strengthening exercises is initiated.
EXTENSOR HALLUCIS LONGUS
It is rare to rupture the extensor hallucis tendon, but such injuries can occur when a sudden plantarflexory force is applied to the extending hallux. More commonly, injuries to the extensor hallucis longus tendon are due to lacerations of the tendon as it traverses the relatively unprotected dorsum of the foot. In fact, this tendon is the most commonly lacerated extensor in lawn mower injuries (Fig. 100.10) (96). Several studies have demonstrated that avulsion injuries at the base of the distal phalanx can occur, but these injuries are rare and are associated with sports that involve running and jumping (97).
In the evaluation of extensor hallucis longus tendon injury, the clinical examination is of key importance. Patients who have sustained extensor hallucis longus injury will demonstrate weakness in extension of the great toe on the effected side. In some instances, the extensor hallucis brevis can generate some active motion of the hallux, and therefore, it is important to rule out motion at the first metatarsophalangeal joint that is due to brevis function. Additionally, the clinician may also notice the loss of the tented appearance of the skin at the at the first metatarsophalangeal joint as compared with the contralateral side, which suggests loss of extensor hood function. Furthermore, extensor tendon pathology is easily evaluated with the use of longitudinal ultrasonography (98,99).
There is much discussion with regard to extensor hallucis longus repair. Some authors have advocated conservative therapy in the treatment of extensor hallucis longus pathology, citing the well-observed phenomenon that extensor tendons tend to heal spontaneously (100,101). Other authors have suggested that surgical repair of the extensor hallucis longus tendon to provide for increased postinjury function should be attempted
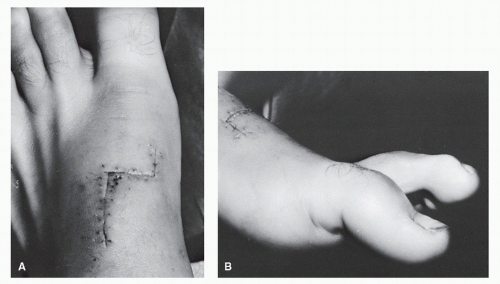
(102,103). In these instances of surgical repair, an end-to-end anastomosis is usually possible when treated early (104). In these instances, nonabsorbable suture is utilized to secure the repair site. When retraction has occurred, a tendon-passing instrument can be introduced proximally into the tendon sheath to draw the proximal stump distally. Patients suffering extensor tendon injury in which there has been a delay in repair or in those instances in which significant retraction has occurred often require tendon grafting to provide for surgical repair (105). In these cases, a free graft from the extensor hallucis brevis may be utilized in either an end-to-end or side-to-side fashion (Fig. 100.11) (106).
(102,103). In these instances of surgical repair, an end-to-end anastomosis is usually possible when treated early (104). In these instances, nonabsorbable suture is utilized to secure the repair site. When retraction has occurred, a tendon-passing instrument can be introduced proximally into the tendon sheath to draw the proximal stump distally. Patients suffering extensor tendon injury in which there has been a delay in repair or in those instances in which significant retraction has occurred often require tendon grafting to provide for surgical repair (105). In these cases, a free graft from the extensor hallucis brevis may be utilized in either an end-to-end or side-to-side fashion (Fig. 100.11) (106).
Following surgical repair of extensor hallucis longus tendon injuries, the patient should be placed in a short-leg, non-weight-bearing cast with the ankle at 90 degrees and the hallux at neutral position or in slight dorsiflexion for approximately 4 weeks (58,104,105). Following this immobilization, the patient should initiate progressive weight-bearing with strengthening exercises. In those cases in which the extensor hallucis has been completely avulsed from the base of the distal phalanx, surgical repair is generally indicated and post repair these patients are placed in a short-leg, non-weight-bearing case for approximately 6 weeks with the hallux in extension (Fig. 100.12).
EXTENSOR DIGITORUM LONGUS
Injury to the extensor tendons of the lesser digits is rare, and as with extensor hallucis longus injuries, injury to the extensor digitorum longus tendons mostly commonly present due to open wounds or lacerations on the dorsum of the foot (104). As previously discussed, physical exam remains an important tool in evaluation of these injuries. Patients suffering from extensor digitorum longus injury will present with weakness in extension of the affected toes. Occasionally, the severed ends of the involved tendons can be palpated within the subcutaneous tissue. As previously discussed, these tendons injuries can be easily evaluated utilizing ultrasonography to confirm the diagnosis.
Management for extensor digitorum longus pathology is largely conservative, with the patient being placed in a shortleg, non-weight-bearing case for approximately 4 weeks; this is especially true for those injuries involving individual segments or terminal branches of the tendon. In those instances in which the entire extensor tendon is ruptured or lacerated, open repair of the tendon may be indicated utilizing techniques that have previously been described (104).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree