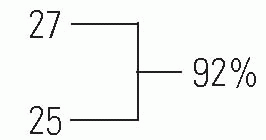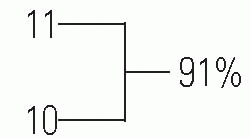Legg-Calvé-Perthes Syndrome
Stuart L. Weinstein
Legg-Calvé-Perthes disease remains one of the most controversial topics in all of pediatric orthopaedic surgery. The debate about its etiology and pathogenesis continues, and there is no unanimity regarding treatment. This chapter reviews what is known about the condition, points out where controversies exist, and highlights the problems in decision making regarding treatment.
EARLY HISTORY
Legg-Calvé-Perthes syndrome is a disorder of the hip in young children. The condition was described independently in 1910 by Legg (1), Calvé (2), Perthes (3), and Waldenstrom (4, 5). In the late 19th century, however, Thomas (6), Baker (7), and Wright (8) described patients with supposed hip joint infections that resolved without surgery, whose histories were consistent with Legg-Calvé-Perthes disease. Maydl (9), in 1897, reported this condition and thought it was related to congenital dislocation of the hip (10). Recent findings, discussed in this chapter’s section on pathogenesis, suggest that Legg-Calvé-Perthes “disease” may more appropriately be called a syndrome.
In 1909, Arthur Legg presented a paper on five children who were limping after injury. This paper was published in 1910. He called this condition an “obscure affectation of the hip” and postulated that pressure secondary to injury caused flattening of the femoral head (1). In that same year, Calvé reported 10 cases of a noninflammatory self-limiting condition that healed with flattening of the weight-bearing surface. He postulated that the cause of this condition was an abnormal or delayed osteogenesis. He reported coxa vara and increased femoral head size in these patients; on physical examination, all of the patients had decreased abduction (2). Perthes simultaneously reported six cases of what he termed “arthritis deformans juveniles.” He postulated that this was an inflammatory condition (3). In his description of the condition, Waldenstrom postulated that the disease was a form of tuberculosis (4, 5).
Perthes was the first investigator to describe the pathologic and histologic features of the disorder (11). He reported on a 9-year-old boy who had experienced symptoms for 2 years. Examination of a portion of the excised head revealed numerous cartilage islands throughout and “strings” connecting the cartilage of the joint and the physeal plate. Perthes noted that the marrow spaces were widened, with fatty infiltration; he saw no evidence of inflammation. He believed that the cartilage islands were new and that this was an osteochondritis and not a tubercular process (11). Schwartz (12), an associate of Perthes, described the pathologic changes in a 7-year-old boy with a 2-year history of symptoms and reported similar findings. Waldenstrom (13) suggested the use of the term coxa plana to make the description of the disease consistent with that of other hip deformities, such as coxa vara and coxa valga. Sundt (14, 15) published the first monograph on Legg-Calvé-Perthes syndrome, reporting on 66 cases and the pathology of the condition. The essential feature in all of his cases was the cartilaginous islands in the epiphysis. Sundt attributed the disease to an “osteodystrophy due to dysendocrinia of a hereditary disposition.” He believed that individuals so predisposed would get Legg-Perthes disease after they sustained an injury (i.e., infection or trauma) to the hip. Sundt was the first to introduce the modern concept of the “susceptible child.”
Phemister (16), reporting on the curettage findings in a 10-year-old child with an 8-month history of symptoms, described areas of bone necrosis, granulation tissue, old bone
with new bone formation, and osteoclasts. He interpreted these findings as an inflammatory and infectious process. In 1922, Riedel (17) reported on two cases and presented the histology. He described the thickening of the articular cartilage and noted that the junction between the bone and the articular cartilage was filled with blood. He also noted that the physeal plate was destroyed and that there were many cartilage rests. Dead bone was surrounded by a rich granulation tissue, and many giant cells were present. He also noted that farther away from the main disease process, the marrow was fibrotic with inflammatory infiltrates. Riedel was the first investigator to notice that there were blastic and clastic changes working at the same time on the same bone trabeculae. In his second specimen, he found regeneration of the cartilage in the subchondral area, cell atrophy, and some inflammatory cells. That same year, Waldenstrom (18) proposed the first radiographic classification of the disease process on the basis of the data from 22 patients who were followed up until the completion of their growth. Since then, most of the orthopaedic literature has centered on the etiologic, epidemiologic, and prognostic factors in Legg-Calvé-Perthes disease and follow-up of various treatment modalities (19, 20, 21, 22 and 23).
with new bone formation, and osteoclasts. He interpreted these findings as an inflammatory and infectious process. In 1922, Riedel (17) reported on two cases and presented the histology. He described the thickening of the articular cartilage and noted that the junction between the bone and the articular cartilage was filled with blood. He also noted that the physeal plate was destroyed and that there were many cartilage rests. Dead bone was surrounded by a rich granulation tissue, and many giant cells were present. He also noted that farther away from the main disease process, the marrow was fibrotic with inflammatory infiltrates. Riedel was the first investigator to notice that there were blastic and clastic changes working at the same time on the same bone trabeculae. In his second specimen, he found regeneration of the cartilage in the subchondral area, cell atrophy, and some inflammatory cells. That same year, Waldenstrom (18) proposed the first radiographic classification of the disease process on the basis of the data from 22 patients who were followed up until the completion of their growth. Since then, most of the orthopaedic literature has centered on the etiologic, epidemiologic, and prognostic factors in Legg-Calvé-Perthes disease and follow-up of various treatment modalities (19, 20, 21, 22 and 23).
EPIDEMIOLOGY AND ETIOLOGY
Legg-Calvé-Perthes syndrome occurs most commonly in the age range of 4 to 8 years (24), but cases have been reported in children from 2 years of age to the late teenage years. It is more common in boys than in girls by a ratio of 4 or 5 to 1 (25). The incidence of bilaterality has been reported as 10% to 12% (24, 26). Although the incidence of a positive family history in patients with Legg-Calvé-Perthes syndrome ranges from 1.6% to 20% (10, 24, 27, 28, 29, 30, 31, 32 and 33), there is currently no evidence that this syndrome is an inherited condition (34, 35).
Wynne-Davies and Gormley (24) reported on a series of 310 index patients with Legg-Calvé-Perthes syndrome. They noted that, of the children of index patients with the syndrome, only 2% had Legg-Calvé-Perthes syndrome. All twins in this series were discordant, including one monozygotic pair. Eleven percent had abnormal birth presentations, including breech and transverse, compared with the 2% to 4% incidence that would be expected in the general population. There is a higher incidence of Legg-Calvé-Perthes syndrome in later-born children, particularly the third to the sixth child, and a higher percentage in lower socioeconomic groups (36, 37). Parents of the children with the syndrome also tend to be older than those in the general population (24, 33, 38).
Legg-Calvé-Perthes syndrome is more common in certain geographic areas, particularly in urban rather than rural communities, giving rise to the suspicion of a nutritional cause, possibly a trace element deficiency (36—44). There is also a reported strong association (33% of patients with the syndrome) of Legg-Calvé-Perthes disease with the psychological profile associated with attention-deficit hyperactivity disorder (45). Malloy and MacMahon (46, 47) as well as Lappin et al. (48) noted that birth weight was lower in children with the syndrome. Harrison et al. (49) reported that children with Legg-Calvé-Perthes syndrome lagged behind their chronologic age, and 89% of the involved individuals had delayed bone age. Ralston (50) and others (51, 52) demonstrated that this delay in skeletal maturation averages 21 months but that during the healing stages of the disease, there was recovery of height and weight through increased growth velocity (35, 50, 52). Race may also be a factor in the frequency of incidence of this condition. There is a higher frequency of occurrence of Legg-Calvé-Perthes syndrome among the Japanese, other Asians, Eskimos, and Central Europeans, and a lower frequency of occurrence among native Australians, Americans, Indians, Polynesians, and persons of African origin (10, 41, 53, 54).
There is considerable evidence of anthropometric abnormalities in children with Legg-Calvé-Perthes syndrome. Cameron and Izatt (55) reported that boys with the syndrome were 1 in. shorter and girls with the syndrome were 3 in. shorter compared with healthy children. Burwell et al. (56, 57 and 58) and others (37, 59, 60) demonstrated that children with the syndrome are smaller in all dimensions, except for head circumference, and shorter in the distal portions of the extremities as opposed to the proximal portions. Loder et al. (61), in a more recent study, demonstrated that bone age of the pelvis in boys was less delayed than that of the hand and wrist. In patients who have the disorder at a young age, the shortness in stature tends to correct during adolescence, whereas patients who have the disorder at an older age tend to be small throughout life (33). Eckerwall et al. (62) followed up 110 children with the disorder in a longitudinal study and showed that these children were shorter at birth, remained short during the entire growth period, and their growth velocity never changed. Burwell et al. (56) demonstrated an abnormality of growth hormone—dependent somatomedin in boys with Legg-Calvé-Perthes syndrome, whereas Tanaka et al. (63), Fisher (27), and Kitsugi et al. (64) reported contrary results.
Growth hormone regulates postnatal skeletal development. The effects of growth hormone on postnatal skeletal development are mediated, in part, by the somatomedins [insulin-like growth factors (IGFs)] (65). Somatomedin C insulin-like growth factor-1 (IGF1) is the principal somatomedin responsible for postnatal skeletal bone maturation (65). Plasma IGF1 levels have been reported to be significantly reduced in children with the disorder during the first 2 years after the diagnosis of Perthes disease. These alterations were accompanied by a tendency toward growth arrest and impaired weight gain. An acceleration in growth and weight gain is believed to accompany the healing stages of the disease, although a recent report by Kealey et al. disputes this growth acceleration following the active stages of the disease (66).
In plasma, nearly all IGF1 is bound to specific binding proteins. However, levels of the major binding protein, insulin-like growth factor-binding protein 3 (IGFBP3), are normal during the first 2 years after the diagnosis of Perthes disease (67, 68). Low levels of circulating IGF1 and failure of IGF1 to increase normally during the prepubertal years in patients with Perthes disease, in conjunction with reportedly normal growth hormone levels, raise the possibility of decreased responsiveness of growth-plate chondrocytes and hepatocytes (65). The combination of moderately reduced IGF1 levels with normal IGFBP3 has been reported in normal-variant short-statured children. The skeletal maturation delay and retarded bone age reported in patients with Perthes disease, in conjunction with the findings described in the preceding text, could be considered to be a retention of the infantile hormone pattern (66).
Malnutrition is one factor that leads to low IGF levels, and this could be related to the reportedly higher incidence of Perthes disease in low-income families (43). The disproportionate skeletal development affecting the distal portions of the body reflects a tendency toward infantile body proportions. This correlates with the reduced IGF1 levels in the presence of normal levels of binding proteins (69). Controversy still exists in that a study by another group of investigators reported results opposite to those reported by Neidel et al. (68), with serum levels of IGF1 being normal and those of IGFBP3 being lower in children with Perthes disease, compared with controls (70). Another recent study, although confirming the skeletal maturation delay in children with Perthes disease, demonstrated no difference in IGF1 (measured with IGF2-blocked binding sites) and IGFBP3 serum concentrations with respect to bone age (71). This group disputed the claims of disturbance of the hypothalamic—pituitary—somatomedin axis in patients with Perthes disease. The reported differences in the various studies, in some cases, may be partly attributable to the methods used for measuring IGF1. There is an increased incidence of hernia in patients with Legg-Calvé-Perthes syndrome and their first-degree relatives. There is also an increased incidence of minor congenital abnormalities in patients with the syndrome (29, 72, 73 and 74).
The cause of Legg-Calvé-Perthes syndrome remains unknown. Many etiologic theories have been proposed. In the early part of the 20th century, most investigators thought that it was a disease of an inflammatory or infectious nature (3, 4 and 5, 75, 76 and 77). Phemister (16, 78) believed that the disease was an infectious process, although tissue cultures were negative. Axhausen (75) believed that it was caused by bacillary embolism in which the infection either was not manifested or was too weak and healed quickly. As late as 1975, Matsoukas (79) demonstrated an association between Legg-Calvé-Perthes syndrome and prenatal rubella.
Until the 1950s, trauma was considered by many investigators to be the cause, or a significant contributing factor, of Perthes disease (1, 80, 81, 82, 83, 84, 85 and 86). As with most childhood orthopaedic conditions, a significant number of patients may relate an episode of trauma to the onset of symptoms.
Many investigators, particularly those from Eastern Europe, thought that Legg-Calvé-Perthes syndrome was of congenital origin, and that there was a relationship between this disease and congenitally dislocated hips (84, 85, 86, 87, 88, 89, 90 and 91). Glimcher (92) proposed that cytotoxic agents of external or endogenous origin may be responsible for bone cell death (93). A recent report showed the association of Perthes with delayed ossification of the proximal femoral epiphysis (94). At one time, Perthes disease was believed to be related to hypothyroidism (95—97); this has since been disproved (89, 90). Recent reports demonstrate moderately increased plasma concentrations of free thyroxin and free triiodothyronine in patients with Perthes disease, compared with controls (69). It has yet to be conclusively determined whether the aforementioned factors contribute to causing Perthes disease, and whether IGF1 is at reduced levels in the early disease stages as reported. These findings do, however, provide additional evidence that growth-related systemic abnormalities exist in patients with Legg-Calvé-Perthes syndrome (69).
Transient synovitis has been thought, by many investigators, to be a precursor to the condition. Gershuni (98) reported that 25% of children with benign transient synovitis developed Legg-Calvé-Perthes disease, whereas Jacobs (99) reported three cases of Legg-Calvé-Perthes disease among 25 patients with acute transient synovitis. Although all hips with Perthes disease have synovitis, especially early in the course of the disease, and many have persistent synovitis for years (100—104), a review of the literature reveals that an average of 1% to 3% of patients with a history of transient synovitis later develop Legg-Calvé-Perthes syndrome (105, 106, 107, 108, 109 and 110). Chuinard (111, 112) and Craig et al. (82, 83) have proposed that excessive femoral neck anteversion is a causative factor in the development of Legg-Calvé-Perthes syndrome.
Most current etiologic theories involve vascular embarrassment. An insufficient blood supply to the proximal femur has been elucidated by many authors. The terminology used in the literature varies. However, there are three main sources of blood to the proximal femur: an extracapsular arterial ring, the ascending cervical (retinacular branches) vessels, and the artery of the ligamentum teres (113) (Fig. 24-1). The extracapsular ring is formed mostly by the medial and lateral femoral circumflex vessels. This ring gives rise to the ascending cervical branches, which are extracapsular, and these in turn give rise to the metaphyseal and epiphyseal branches. The anterior portion of the extracapsular ring is formed primarily by the lateral femoral circumflex artery. The posterior, lateral, and medial aspects of the ring are formed by the medial femoral circumflex artery. Chung (113) found that the greatest volume of blood flow to the femoral head comes through the lateral ascending cervical vessel (the termination of the medial femoral circumflex artery), which crosses the capsule in the posterior trochanteric fossa. Trueta and Pinto de Lima (114, 115) and Chung (113) demonstrated that the anterior vascular anastomotic network (Fig. 24-1) is much less extensive than the
posterior anastomotic network, particularly in specimens taken from patients 3 to 10 years of age, which correlates with the age range of Legg-Calvé-Perthes syndrome. Chung (113) also demonstrated that the anterior anastomotic network was incomplete more often in boys, which correlates with the male predominance found in Legg-Calvé-Perthes syndrome. Ogden (116) reported the presence of vessels crossing the physeal plate in some of his specimens, but Chung disagreed, suggesting instead that the vessels do not actually cross the plate, but pass through the peripheral perichondral fibrocartilaginous complex.
posterior anastomotic network, particularly in specimens taken from patients 3 to 10 years of age, which correlates with the age range of Legg-Calvé-Perthes syndrome. Chung (113) also demonstrated that the anterior anastomotic network was incomplete more often in boys, which correlates with the male predominance found in Legg-Calvé-Perthes syndrome. Ogden (116) reported the presence of vessels crossing the physeal plate in some of his specimens, but Chung disagreed, suggesting instead that the vessels do not actually cross the plate, but pass through the peripheral perichondral fibrocartilaginous complex.
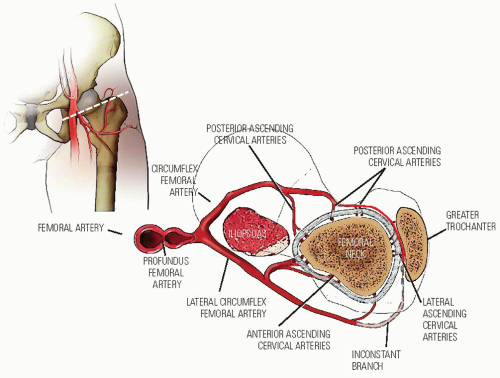 FIGURE 24-1. The blood supply to the normal proximal femur in a child. (From Chung SMK. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg Am 1976;58:961.) |
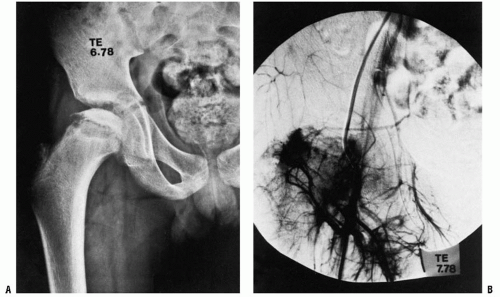
Interruption of the blood supply to the femoral head in Perthes disease was first demonstrated in 1926, when Konjetzny (77) showed obliterative vascular thickening in a pathologic specimen. Theron (117) used selective angiograms to demonstrate obstruction of the superior retinacular artery in patients with Legg-Calvé-Perthes syndrome. In a recent angiographic study, Atsumi et al. (118) showed that 68% of subjects with Perthes disease had interruption of the lateral epiphyseal arteries at their origin. In 1973, Sanchis et al. (119) proposed the second infarction theory. They experimentally infarcted the femoral head of animals labeled with tetracycline. They were unable to produce a typical histologic picture of Legg-Calvé-Perthes syndrome with only a single infarction. With a second infarction, however, they were able to show a more characteristic histologic picture of Legg-Calvé-Perthes syndrome. Inoue et al. (120, 121) later correlated this doubleinfarction theory with human histologic material. Clinical correlation for this theory is provided by reports of recurrent Perthes disease (122, 123) (Fig. 24-2). Salter and Thompson (124, 125) proposed that Legg-Calvé-Perthes syndrome is a complication of aseptic necrosis, and that a fracture manifested radiographically by a subchondral radiolucent zone initiates the resorptive phase. Kleinman and Bleck (126) demonstrated increased blood viscosity in a group of patients with Legg-Calvé-Perthes syndrome, possibly leading to decreased blood flow to the femoral epiphysis. Vascular embarrassment, caused by intraosseous venous hypertension and venous obstruction, has been demonstrated by several authors (34, 127, 128).
Recently, attention has been centered on reports of protein C and S deficiencies in patients with Perthes syndrome (129, 130, 131, 132, 133, 134, 135, 136 and 137). Thrombophilia induced by low levels of protein C or protein S, or by resistance to activated protein C, has been associated with the development of osteonecrosis and with arterial thrombosis (129, 130, 133, 134, 135, 136 and 137). These investigators have suggested routine screening of the levels of protein C, protein S, and lipoprotein(s); plasminogen activator inhibitor activity; and stimulated tissue plasminogen activator activity in patients with Perthes syndrome (130). They believe that routine coagulation screening of children with Legg-Perthes disease has an additional advantage because of the familial nature of the autosomal dominant coagulopathies. These disorders are associated with thrombotic events in 60% of adult
family members. The authors believe that the diagnosis of a coagulation disorder in a child with Legg-Perthes disease can and should lead to studies in first-degree relatives, with the goal of preventing thrombotic events in families. More recent literature has refuted the role of thrombophilia in causing Perthes disease (138, 139, 140, 141 and 142).
family members. The authors believe that the diagnosis of a coagulation disorder in a child with Legg-Perthes disease can and should lead to studies in first-degree relatives, with the goal of preventing thrombotic events in families. More recent literature has refuted the role of thrombophilia in causing Perthes disease (138, 139, 140, 141 and 142).
PATHOGENESIS
The histologic changes seen in Legg-Calvé-Perthes syndrome should be put in perspective. Few human specimens have been studied, and each such specimen represents only one stage in the disease process. Most specimens are from curettage or core
biopsies, which show only one portion of the involved head at a time.
biopsies, which show only one portion of the involved head at a time.
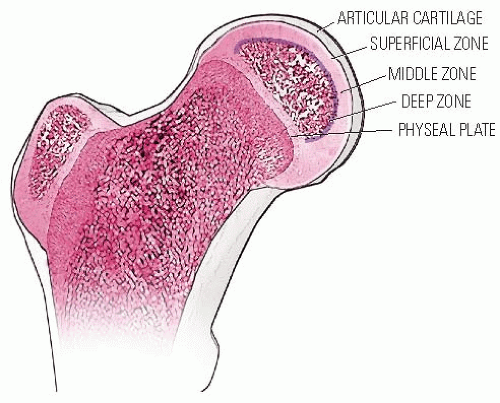
In the developing normal human femoral head, the secondary center of ossification is covered by cartilage comprising three zones (Fig. 24-3). The superficial zone has the morphologic properties of adult articular cartilage. Beneath this zone is the zone of epiphyseal cartilage, which is histochemically different. The zone becomes thinner as the skeleton matures and the epiphyseal bone enlarges in size. Underneath the epiphyseal cartilage is a thin zone formed by small clusters of cartilage cells that hypertrophy and degenerate. Capillaries penetrate this zone from below, and bone forms at a much slower rate than in the metaphysis (143).
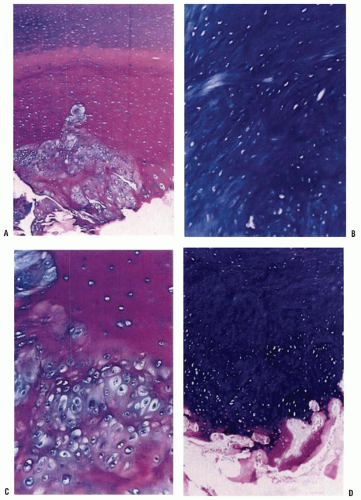
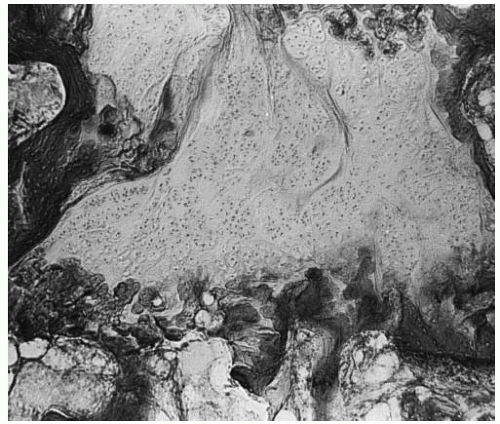
Histologic changes of the epiphyseal and physeal cartilage of patients with Legg-Calvé-Perthes syndrome (Figs. 24-4 and 24-5) were described as early as 1913. These and current studies demonstrate that the superficial zone of the epiphyseal cartilage covering the affected femoral head is normal but thickened. In the middle layer of the epiphyseal cartilage, however, two types of abnormalities are seen: areas of extreme hypercellularity, with the cells varying in size and shape and often arranged in clusters, and areas containing a loose fibrocartilage-like matrix. These abnormal areas in the epiphyseal cartilage have histochemical and ultrastructural properties that are different from normal cartilage and fibrocartilage. Areas of small secondary ossification centers are evident, with bony trabeculae of uneven thickness forming directly on the abnormal cartilage matrix (143, 144, 145, 146, 147 and 148). The superficial and middle layers of epiphyseal cartilage are nourished by synovial fluid and continue to proliferate, whereas only the deepest layer of the epiphyseal cartilage is dependent on the epiphyseal blood supply and is affected by the ischemic process (143, 146, 149, 150, 151, 152, 153, 154, 155, 156 and 157).
The physeal plate also is abnormal in Legg-Calvé-Perthes syndrome. It shows evidence of cleft formation with amorphous debris and extravasation of blood. In the metaphyseal region, endochondral ossification is normal in some areas, but
in others the proliferating cells are separated by a fibrillated cartilaginous matrix that does not calcify (Fig. 24-5). The cells in these areas do not degenerate but continue to proliferate without endochondral ossification, leading to tongues of cartilage extending into the metaphysis as bone growth proceeds in the adjoining areas (143, 144, 148, 158, 159 and 160).
in others the proliferating cells are separated by a fibrillated cartilaginous matrix that does not calcify (Fig. 24-5). The cells in these areas do not degenerate but continue to proliferate without endochondral ossification, leading to tongues of cartilage extending into the metaphysis as bone growth proceeds in the adjoining areas (143, 144, 148, 158, 159 and 160).
Catterall et al. (158) have demonstrated thickening, abnormal staining, sporadic calcification, and diminished evidence of ossification in the deep zone of the articular cartilage of the unaffected hip. They also demonstrated that the physeal plate in these unaffected hips is thinner than normal, with irregular cell columns and cartilage masses remaining unossified in the primary spongiosa.
Some of these cartilage changes have been seen in other epiphyseal plates, such as the greater trochanter and the acetabulum (161). In the human specimens described by Ponseti (144), the physeal plate lesions were longstanding, as shown by the fact that there was only necrotic bone in the femoral head and no evidence of repair. Catterall et al. reported similar cartilaginous lesions in a patient with Catterall group 1 disease, in which there is no sequestrum formation (146, 148) (Fig. 24-6). The various reported physeal plate and epiphyseal plate lesions resemble the lesions that Ponseti and Shepard (162) produced in rats by administering aminonitrils. These epiphyseal and physeal plate changes, in conjunction with the unusual and precarious blood supply to the proximal femur, make the femoral head vulnerable to the effects of physeal plate disruption.
Surveys of patients with Legg-Calvé-Perthes syndrome confirm that the histologic abnormalities are accompanied by irregularities of ossification in other epiphyses, especially Kohler disease of the navicular (73, 144, 163). Harrison and Blakemore (164), studying 153 consecutive patients with unilateral Legg-Calvé-Perthes disease, found that 48% had contour irregularities in the contralateral normal capital epiphysis compared with 10% of the matched controls. Kandzierski et al. (152) reported that 35% of patients with Perthes disease showed changes in the unaffected proximal femur in the first radiograph. Aire et al. (165) demonstrated that the unaffected hip showed anterior and lateral flattening at the time of diagnosis of the affected hip. These data suggest that Legg-Calvé-Perthes disease is a generalized process affecting other epiphyses and therefore should not be referred to as a disease but should be called Legg-Calvé-Perthes syndrome.
Disorganization of the physeal plate, together with minimal trauma, may interrupt the continuity of retinacular vessels, causing necrosis (143, 144). This finding, in conjunction with the aforementioned epidemiologic, histologic, and radiologic data, supports the belief that Legg-Calvé-Perthes syndrome may be a localized manifestation of a generalized disorder of epiphyseal cartilage in the susceptible child (10, 34, 52, 59, 72, 143, 151, 166, 167).
Radiographic Stages.
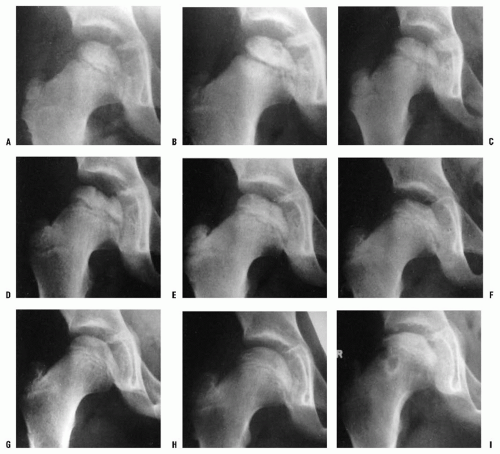
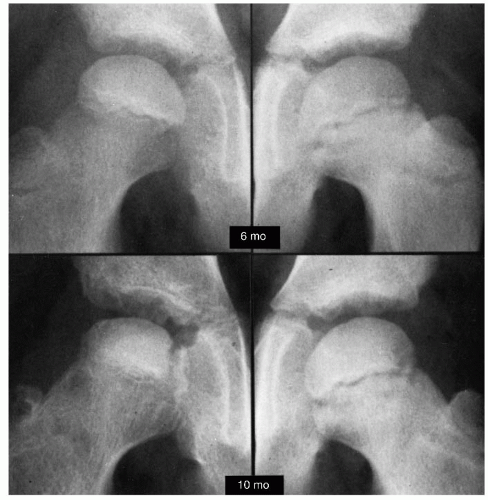
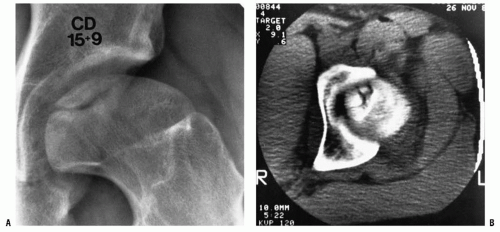
Radiographically, Legg-Calvé-Perthes syndrome can be classified into four stages: initial, fragmentation, reossification, and healed. These stages are important in the formulation of treatment decisions that will be discussed later in this chapter. In the initial stage (18, 168), one of the first signs of this condition is failure of the femoral ossific nucleus to increase in size because of a lack of blood supply (Fig. 24-7). The affected femoral head appears smaller than the opposite, unaffected ossific nucleus. Widening of the medial joint space, as initially described by Waldenstrom (18, 169) (Fig. 24-7), is another early radiographic finding. Some researchers have theorized that widening is caused by synovitis. Others have proposed that this finding is secondary to decreased head volume caused by necrosis and collapse and a secondary increase in blood flow to the soft-tissue parts, such as the ligamentum teres and pulvinar, causing the head to displace laterally (168, 170). Synovitis is indeed present in patients with Perthes disease to varying degrees (101—104, 171, 172), but the medial joint space widening is probably most often an apparent radiographic phenomenon secondary to epiphyseal cartilage hypertrophy (Fig. 24-8).
In the initial stage, the physeal plate is irregular and the metaphysis is blurry and radiolucent (87) (Fig. 24-9). The femoral ossific nucleus appears radiodense (173). This relative increase in radiodensity may be caused by osteopenia of the surrounding bone (174, 175) or an increase in the mass of bone per unit area.
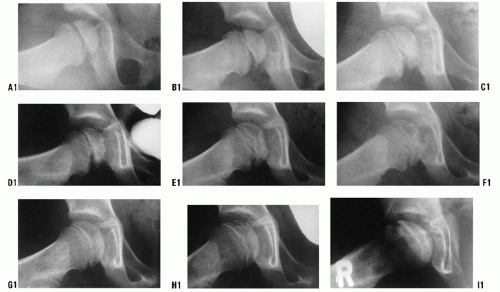
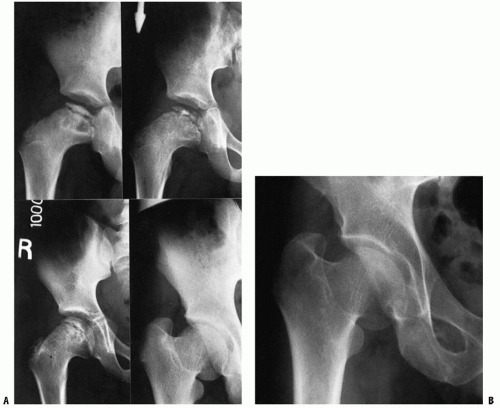
The second radiographic stage is called the fragmentation phase (18, 168). Radiographically, the repair aspects of the disease become more prominent (Fig. 24-9). The bony epiphysis begins to fragment, and there are areas of increased radiolucency and increased radiodensity. Increased radiodensity at this stage may be caused by new bone forming on old bone (176, 177, 178, 179 and 180) and thickening of existing trabeculae (178). The subchondral radiolucent zone (i.e., crescent sign) first described by Waldenstrom (169, 181), and later brought to wider attention by Caffey (182), is one of the very early signs
of Legg-Calvé-Perthes syndrome in the fragmentation stage (Figs. 24-2 and 24-10). According to Salter and Thompson (125) and Salter and Bell (183), this radiographic finding results from a subchondral stress fracture, and the extent of this zone determines the extent of the necrotic fragment.
of Legg-Calvé-Perthes syndrome in the fragmentation stage (Figs. 24-2 and 24-10). According to Salter and Thompson (125) and Salter and Bell (183), this radiographic finding results from a subchondral stress fracture, and the extent of this zone determines the extent of the necrotic fragment.
The third radiographic stage is the reparative or reossification phase (18, 168). Radiographically normal bone density returns, with radiodensities appearing in areas that were formerly radiolucent. Alterations in the shape of the femoral head and neck become apparent (Fig. 24-9).
The final stage is the healed phase. In this stage, the proximal femur may have residual deformity from the disease and the repair process (Fig. 24-9).
In the young child, Legg-Calvé-Perthes syndrome cannot be compared with aseptic necrosis after fracture of the neck of the femur or traumatic dislocations of the hip. In these
situations, the vascular insult to the femoral head usually heals rapidly without going through the prolonged stages of fragmentation and repair that are seen in children with Legg-Calvé-Perthes syndrome (143, 184, 185).
situations, the vascular insult to the femoral head usually heals rapidly without going through the prolonged stages of fragmentation and repair that are seen in children with Legg-Calvé-Perthes syndrome (143, 184, 185).
Pathogenesis of Deformity.
The deformities of the femoral head that occur in Legg-Calvé-Perthes syndrome come about in many ways. First, there is growth disturbance in the epiphyseal and physeal plates. In the physeal plate, this may result in premature closure with resultant deformity, such as central physeal arrest, causing shortening of the neck of the femur and trochanteric overgrowth (186, 187) (Fig. 24-11). The repair process itself may cause physical compaction resulting from structural failure and displacement of tissue elements (92). During the healing process, the femoral head will deform according to the asymmetric repair process and the
applied stresses. The molding action of the acetabulum during new bone formation may also play a role in this process (188, 189). With deformity of the femoral head, the acetabulum, particularly its lateral aspect, is deformed secondarily.
applied stresses. The molding action of the acetabulum during new bone formation may also play a role in this process (188, 189). With deformity of the femoral head, the acetabulum, particularly its lateral aspect, is deformed secondarily.
The articular cartilage of the femoral head shows changes in shape secondary to the disease process itself. The deepest layer of the articular cartilage is nourished by the subchondral blood supply. This layer is often devitalized in Legg-Calvé-Perthes syndrome (146, 149, 150, 152, 153 and 154, 156). The superficial layers that are nourished by synovial fluid continue to proliferate, causing an increase in the thickness of the articular cartilage. With trabecular collapse and fracture and articular cartilage overgrowth, significant femoral head deformities develop that are manifested clinically as loss of abduction and rotation (Fig. 24-8).
The source of vessel ingrowth is under debate. Many investigators (149, 150, 190) have demonstrated that the new blood vessels arise from the metaphysis and the metaphyseal periosteum and penetrate between the epiphysis and the joint cartilage into the epiphysis. Other investigators have shown metaphyseal vessels penetrating the physeal plate into the epiphysis (112, 116). When the blood supply of the subchondral area is restored, it generally comes from the periphery and moves to the center, first restoring endochondral ossification at the periphery and causing asymmetric growth (156, 191) (Fig. 24-12). In addition, there is abnormal ossification of the disorganized matrix of the epiphyseal cartilage. Finally, there is periosteal bone growth and reactivation of the physeal plate along the femoral neck, with abnormally long cartilage columns leading to coxa magna and a widened femoral neck (143, 144).
The actual deformity that develops is profoundly influenced by the duration of the disease. This, in turn, is proportional to the extent of the epiphyseal involvement, the age of the patient at the time of onset of the disease, the remodeling potential of the patient, and the stage of disease when treatment is initiated. An additional factor is the type of treatment chosen (192, 193, 194 and 195).
Patterns of Deformity.
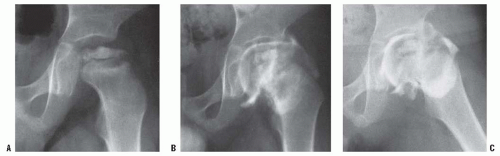
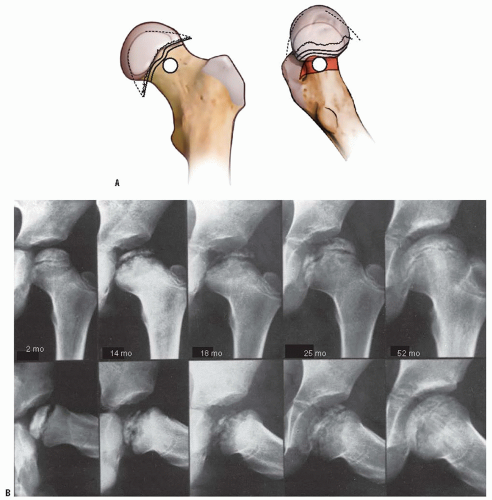
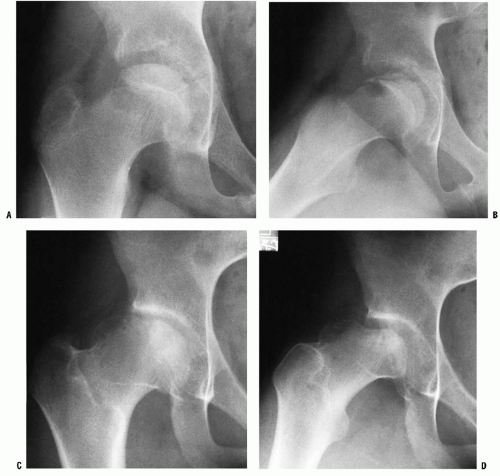
Four basic patterns of residual deformity result from Legg-Calvé-Perthes syndrome: coxa magna, premature physeal arrest patterns, irregular femoral head formation, and osteochondritis dissecans (187, 196). Coxa magna (Fig. 24-9) develops with ossification of the hypertrophied articular cartilage and also from reactivation of the physeal plate along the femoral neck. This also occurs in conjunction with periosteal new bone formation along the femoral neck.
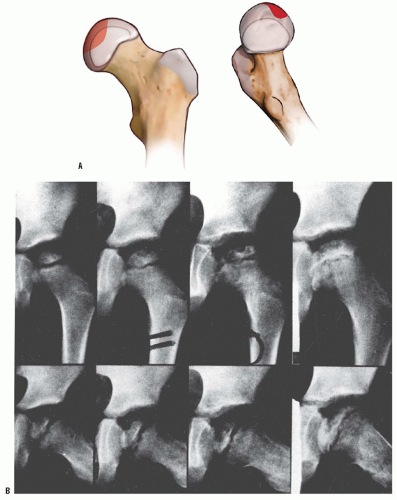
Premature physeal plate closure generally leads to one of two patterns of arrest: central or lateral. In the central arrest pattern, the femoral neck is short and the epiphysis is relatively round (Fig. 24-11). There is trochanteric overgrowth and mild
acetabular deformity. In the lateral arrest pattern, the femoral head is tilted externally (Fig. 24-13). There is also trochanteric overgrowth. The epiphysis is oval, with a corresponding acetabular deformity (187, 196).
acetabular deformity. In the lateral arrest pattern, the femoral head is tilted externally (Fig. 24-13). There is also trochanteric overgrowth. The epiphysis is oval, with a corresponding acetabular deformity (187, 196).
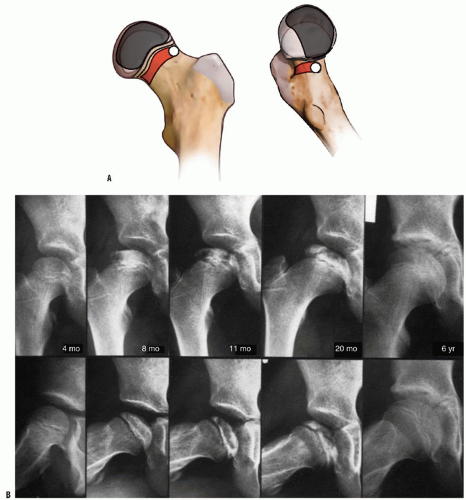
The irregular femoral head may occur as a consequence of certain patterns of physeal arrest, or it may be an iatrogenic deformity from attempts at “containment” of a noncontainable head (Fig. 24-14). After the femoral head becomes deformed and is no longer containable within the acetabulum, the only motion that is allowed is in the flexion and extension plane, with abduction leading to hinging on the lateral edge of the acetabulum. This hinge abduction causes acetabular deformity, leading to femoral head deformity (197, 198 and 199).
NATURAL HISTORY
The formulation of disease treatment requires that the treating physician knows what would happen to the patient in the absence of treatment (natural history) and what factors prognosticate an adverse outcome. The treating physician must determine which of these adverse prognostic factors can be affected by treatment. A treatment plan is then initiated, and long-term follow-up determines whether treatment favorably alters the course of the disease over the long term. The fundamental problem in developing treatment plans for patients with Legg-Calvé-Perthes syndrome is the paucity of natural history data (72, 203, 204, 205 and 206).
Catterall (203) compared 46 untreated hips of Murley and Lloyd-Roberts with a matched control group of 51 hips treated with a weight-relieving caliper. The average age at diagnosis was 4 years and 6 months, and the average follow-up was 10 years and 5 months, with a range of 4 to 18 years. The patients were evaluated according to the grading system of Sundt (121), which requires some subjective assessments. The 10-year average follow-up in this series was too short to determine the outcomes for patients and thus the natural history of the disease, because most patients with childhood hip disease do well regardless of the radiographic appearance in their early years (207—210). In addition, no data are presented on the interobserver or intraobserver reliability of the outcome criteria.
Catterall (203) also reported on 97 untreated patients from around the British Isles (203). The average follow-up in this series was only 6 years, and the results were graded according to the aforementioned system of Sundt (121). The outcomes in this group of patients (Table 24-1) are widely quoted in the literature as a comparison for outcomes of various treatment modalities. Unfortunately, very few articles in the literature use the same grading system for outcomes, and the follow-up of this group is too short to be defined as natural history.
The only other article labeled “natural history” in the literature (206) is not a natural history study but a study of patients from three centers treated by different methods. This study attempted both to establish a relation between residual deformity and degenerative joint disease and to identify clinical and radiographic factors in the active phase of disease that would be predictive of hip deformity and degenerative joint disease. Therefore, as will be further discussed later in the chapter, decision making with reference to treatment is difficult because of the lack of true long-term natural history data.
Long-Term Follow-Up Results.
Although there is little information available on natural history, there are many longterm follow-up studies of patients with Legg-Calvé-Perthes syndrome. The long-term studies that are available suffer from the faults of retrospective long-term reviews in that most series contain only small numbers of patients, with many of the original patients not traced; original radiographs often are not available. Many of the longer series contain patients diagnosed in the years 1910 to 1940, when little was known about the disease, prognostic factors, and radiographic classifications. In most series, patients are combined regardless of what are now known to be prognostic factors: the extent of epiphyseal involvement, age at onset of the disease, age at the beginning of treatment, and stage of the disease at treatment initiation. Various treatment modalities are combined in many series, and control groups are generally absent. Because of these inherent problems, and the fact that different grading systems are used in judging clinical and radiographic end results, all of which lack interobserver and intraobserver reliability data, it is difficult to compare and contrast the various reported series. Despite these shortcomings, a great deal has been learned about the prognosis in Legg-Calvé-Perthes syndrome.
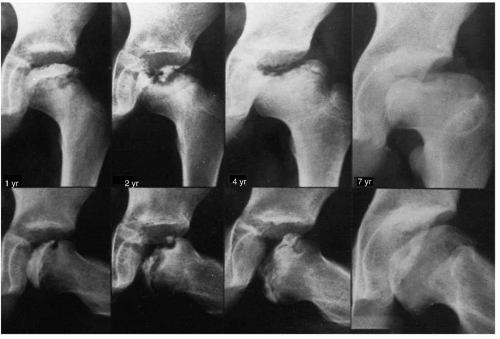
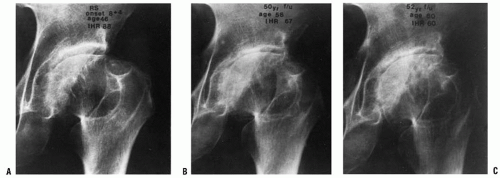
In reviewing long-term follow-up studies, it is apparent that results can improve with time, because remodeling potential continues until the end of growth (72, 211) (Figs. 24-9 and 24-10). Mose wrote that, “for a precise prognosis, conclusions from any measurements ought not be made before the patient reaches the age of 16, when growth stops” (209). Reviews of the outcomes of treatment modalities before skeletal maturity must be viewed as preliminary reports.
Twenty to forty years after the onset of symptoms, most (70% to 90%) patients with Legg-Calvé-Perthes syndrome are active and free of pain. Most patients maintain a good range of motion, despite the fact that few have normal-appearing radiographs. Clinical deterioration and symptoms of increasing pain, decreasing range of motion, and loss of function
are observed only in patients with flattened irregular heads at the time of primary healing, and in patients with premature physeal closure, as indicated by femoral neck shortening, femoral head deformity, and trochanteric overgrowth (193) (Fig. 24-13).
are observed only in patients with flattened irregular heads at the time of primary healing, and in patients with premature physeal closure, as indicated by femoral neck shortening, femoral head deformity, and trochanteric overgrowth (193) (Fig. 24-13).
Danielsson and Hernborg (212) reported a 33-year follow-up of 35 patients. Twenty-eight of the thirty-five patients were free of pain, with 34 of 35 functioning without restrictions. In a 34-year follow-up, Hall (213) reported satisfactory results in 71% of 209 cases. Perpich et al. (214) reported a 30-year follow-up of 37 patients. The average Iowa Hip Rating was 93 of a possible 100 points. Eighty-five percent of the patients had good clinical results, despite the fact that only 33% had spherical femoral heads, as rated by the Mose Sphericity Scale (209) (Fig. 24-16). Forty-three percent of the patients had poor Mose ratings; however, of these patients, 76% had good clinical results.
Ratliff (215) followed 34 patients for an average of 30 years and noted that 80% were fully active and free of pain, whereas only 40% were radiographically normal. He
followed 16 of these patients for an additional 11 years (216) and noted that, despite the fact that only one-third of them had good anatomic results, “deterioration rarely occurred and many patients had no pain and (maintained) normal activity.”
followed 16 of these patients for an additional 11 years (216) and noted that, despite the fact that only one-third of them had good anatomic results, “deterioration rarely occurred and many patients had no pain and (maintained) normal activity.”
Yrjonen (210) followed 96 patients (106 hips), all of whom had noncontainment treatment, for an average of 35 years. At maturity, 61% had poor results by the Mose criteria. In a final follow-up, 48% had evidence of degenerative joint disease. However, at an average 35-year follow-up, only 4% had undergone total hip arthroplasty, with an additional 13% having clinical symptoms significant enough to warrant arthroplasty. Ippolito et al. (217) reported on 61 patients with an average follow-up of 25 years. Only 19% of their patients had poor results, as measured by the Iowa Hip Rating, at final follow-up. W.J. Cumming (personal communication, 1997) reported on 82 patients with 95 involved hips treated by prolonged frame recumbency, with an average follow-up of 38 years. Only 10% of the patients had required arthroplasty at follow-up, with an additional 10% having symptoms significant enough to warrant arthroplasty.
TABLE 24-1 Results for 97 Untreated Hips | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Gower and Johnston (218) reported on 30 nonoperated hips with an average 36-year follow-up. This series is representative of other 20-to 40-year long-term series reported in the literature. The average Iowa Hip Rating for these 30 patients was 91 points. The typical patient had minimal shortening, absent or mild hip pain, and minimal or no functional impairment with respect to their jobs and activities of daily living. Ninety-two percent of the patients had Iowa Hip Ratings higher than 80 points, and only 8% of them had undergone arthroplasty.
In follow-up studies beyond 40 years, hip function begins to deteriorate. In another study of the Iowa group of patients at 48-year follow-up, McAndrew and Weinstein (208) reported that only 40% of patients maintained an Iowa Hip Rating of better than 80 points. Forty percent of the patients had undergone arthroplasty, and an additional 10% had disabling osteoarthritis symptoms but had not yet undergone arthroplasty (Fig. 24-17). Further, at 48-year follow-up, 50% of the patients had disabling osteoarthritis and pain, and an additional 10% had Iowa Hip Ratings of <80 points. The prevalence of osteoarthritis in this group of patients was ten times that found in the general population in the same age range (193). Mose followed a group of patients into the seventh decade of life. All of the patients with irregular femoral
heads had degenerative arthritis. Of those patients with femoral heads that Mose classified as “normal, ball shaped,” no patient had degenerative joint disease by the middle of the fourth decade, but 67% had severe degenerative arthritis by the middle of the seventh decade (209). Therefore, the follow-up studies beyond 40 years demonstrate marked reduction of function, with most of the patients developing degenerative joint disease by the sixth and seventh decades (187, 206, 207, 208 and 209).
heads had degenerative arthritis. Of those patients with femoral heads that Mose classified as “normal, ball shaped,” no patient had degenerative joint disease by the middle of the fourth decade, but 67% had severe degenerative arthritis by the middle of the seventh decade (209). Therefore, the follow-up studies beyond 40 years demonstrate marked reduction of function, with most of the patients developing degenerative joint disease by the sixth and seventh decades (187, 206, 207, 208 and 209).
Prognostic Factors.
In reviews of long-term series of patients with Legg-Calvé-Perthes syndrome, certain clinical and radiographic features have been identified that have prognostic value (193, 197, 210, 219, 220, 221, 222 and 223) (Table 24-2). The most important prognostic factor in determining the outcome is the residual deformity of the femoral head, coupled with hip joint incongruity (224, 225 and 226). Femoral head deformity and joint incongruity are multifactorial problems. They are interrelated with all of the other prognostic factors. It must be kept in mind that Legg-Calvé-Perthes syndrome represents a growth disturbance of the proximal femur; the epiphyseal and physeal cartilage is abnormal. Other key factors involved in the development of deformity include the extent of epiphyseal involvement and the varying degrees and patterns of premature physeal closure associated with this condition (227).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree