Laminectomy and Posterior Fusion
Paul A. Anderson
Michael A. Finn
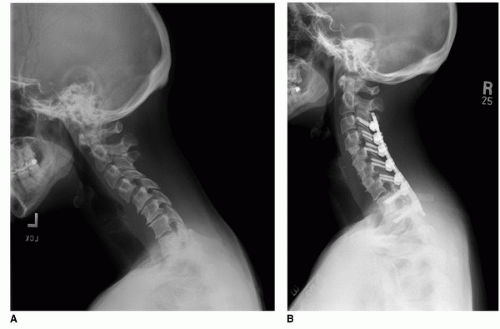
Posterior cervical decompression and fusion is a commonly performed surgery used to treat neurologic compression and spinal instability. Cervical laminectomy, once popular, has diminishing utilization as a stand-alone procedure secondary to its potential to lead to kyphotic deformity and recurrent symptoms (Fig. 10-1) (8,9). Early attempts at stabilization after laminectomy with wiring constructs were fraught with difficulty, required rigid postoperative orthoses, and had a poor capacity to maintain correction. Modern lateral mass and pedicle screw instrumentation has, however, enabled stable reconstruction of the unstable spine even after laminectomy. These newer stabilization techniques are simpler to implement and safer, have better clinical results, and decrease the need for postoperative orthoses when compared with prior methods of fusion.
INDICATIONS
Posterior laminectomy and fusion is indicted when posterior decompression is required for the treatment of spinal cord compression syndromes in combination with instability or kyphotic deformity. Most commonly, this is for the treatment of cervical spondylotic myelopathy associated with degenerative spondylolisthesis or kyphotic deformity. Other conditions include intradural pathology (e.g., spinal cord tumor) needing to be accessed and the potential for the creation of iatrogenic instability (22,31). In some cases of traumatic central cord syndrome requiring decompression, fusion is added if there is concomitant discoligamentous injury (Table 10-1).
There is currently no consensus on the definition of cervical instability. Although rigid radiographic criteria for cervical instability have been proposed, including more than 3.5 mm of subluxation and 11 degrees of angulation on dynamic x-rays (28), more broadly encompassing definitions are often cited. White and Panjabi defined clinical instability of the spine as the loss of the spine’s ability to maintain its patterns of displacement under physiologic loads so there is no initial or additional neurologic deficit, no major deformity, and no incapacitating pain (27). Broader definitions such as this extend the indications for fusion to patients with significant axial neck pain and/or deformity who do not present with mobile listhesis.
In cases of cervical spondylotic myelopathy, it is postulated that both motion and compression are contributors to the myelopathic process (30). As such, a fusion may be more strongly considered for this indication. A recent systematic review of the literature did suggest that there is strong evidence to support the utilization of cervical laminectomy and fusion in the treatment of cervical spondylotic myelopathy (1). In cases of cervical kyphosis, fusion should be performed with a goal of reestablishing at least neutral alignment. Irreducible kyphosis may be better treated through an anterior or combined approach.
Advantages of a posterior approach over an anterior approach include surgeon familiarity, ease of exposure, shorter operative times, and reduced risk of dysphagia and recurrent laryngeal nerve palsy (14). Patients with multilevel disease are particularly well suited for posterior approaches as well as patients with ossification of the posterior longitudinal ligament (OPLL), who are at greater risk for complications, including cerebrospinal fluid (CSF) leak and neurologic injury, with an anterior approach (1). The disadvantages of the posterior laminectomy and fusion approach are
the limited area for bone graft placement, need for costly instrumentation, and higher risk of infection.
the limited area for bone graft placement, need for costly instrumentation, and higher risk of infection.
CONTRAINDICATIONS
Posterior decompression and fusion is contraindicated as a stand-alone procedure in cases of significant anterior compressive pathology or in patients with irreducible kyphosis. Relative contraindications include patients at particularly high risk for infection (e.g., s/p radiation, malnutrition, prior posterior approach) who may be adequately treated through an anterior approach.
TABLE 10-1 Indications for Posterior Cervical Fusion | ||||||
|---|---|---|---|---|---|---|
|
TECHNIQUE
Anesthesia
The anesthesiologist must be made aware of the nature of the patient’s condition. In cases with significant canal stenosis, specific anesthetic considerations include intubation, positioning, and hemodynamic support, each of which may compromise the patient’s neurologic integrity. Intubation should be performed with in-line stabilization of the neck, and we recommend consideration of awake endotracheal intubation. This has the advantage of allowing for confirmation of neurologic stability after endotracheal tube placement. Care should be taken to avoid neck hyperextension in patients with significant stenosis, as this may contribute to further canal narrowing and neurologic injury. In myelopathic and significantly stenotic patients, careful monitoring of blood pressure is essential, and intra-arterial lines for continuous blood pressure monitoring are routinely used. We maintain a mean arterial pressure of greater than 85 mm Hg to ensure cord perfusion. The use of multimodal neural monitoring is controversial and may allow for early recognition of neurologic injury (21). Similarly, the use of steroids to prevent spinal cord deterioration is controversial and unproven but is an option in patients with severe spinal stenosis or with neurologic deficits.
Positioning
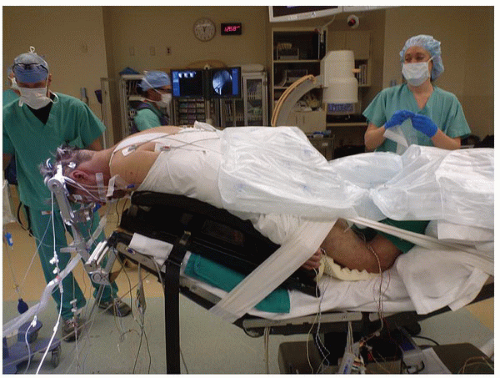
Patients are typically placed in Mayfield 3-point fixation to provide stable positioning without the risk of ocular pressure. Alternatively, patients may be positioned on a padded horseshoe with Gardner-Wells tongs to provide distraction. Care must be taken to ensure that the weight of the head is not resting on the eyes when utilizing this method.
After placement of pins, the patient is rotated into the prone position onto padded gel rolls, a Wilson frame, or the Jackson table. A Jackson table is preferred for obese patients, as it allows for greater decompression of the abdominal compartment and subsequently less venous hypertension and potential for blood loss (Fig. 10-2). A vertical footboard is used to prevent downward sliding when inclining the bed, and the position of the head is fixed. The bed is placed in a reverse Trendelenburg position to aid venous drainage and reduce intraoperative bleeding. However, maintenance of mean arterial pressure after positioning is essential to prevent cord ischemia. Hyperextension is avoided in stenotic patients, as this reduces canal diameter and may predispose to injury. The head is typically positioned in a slight “military tuck” position, with the head flexed and the neck somewhat extended to increase exposure but maintain cervical lordosis. The arms are secured by the patient’s sides, and the shoulders are taped slightly inferiorly to aid in x-ray visualization of lower cervical levels.
Once secured, fluoroscopy can be used to demonstrate adequacy of positioning and maintenance of reduction if alignment is a concern. If neural monitoring is used, signals are rechecked after final positioning.
Exposure
The incision is marked utilizing landmarks, with the C2 and C7 spinous processes easily palpable in most patients. Intraoperative radiographs can aid in planning incisions for small operations.
A midline incision is created and dissected to the fascia with electrocautery. The midline avascular raphe is exploited to minimize blood loss. A self-retaining retractor is placed to maintain exposure when the spinous processes are encountered. Electrocautery is used to create a paramedian fascial incision on either side of the bifid processes. The paraspinous muscles are then elevated off the spinous process and lamina in a subperiosteal fashion, using a Cobb elevator to hold retraction and electrocautery to separate tissue adhesions and maintain hemostasis. Care is taken to avoid damaging the interspinous ligaments, particularly at the cephalad and caudal ends of the planned construct (24). Care is also taken to preserve the joint capsules of the facets at the distal ends of the planned construct. After final exposure, levels are confirmed radiographically. Unless absolutely required for adequate decompression, the nuchal ligaments attachments to C2 and C7 should be preserved or repaired to avoid the development of postoperative kyphosis.
Decompression
The laminectomy can be performed primarily with rongeurs. The cephalocaudal limits of the decompression are demarcated by using a Leksell rongeur to remove the supraspinous and interspinous ligaments. Likewise, these ligaments are removed at each level to be decompressed to facilitate processing of local autograft. Laminectomy can then be performed using a combination of Leksell and Kerrison rongeurs. In cases of severe stenosis, a “no touch technique” (no canal intrusion with instruments) should be employed. In this case, the lamina-facet junction is identified and marked with the marking pen, and a high-speed burr is used to osteotomize the lamina at the lamina-facet junction bilaterally. The trough is cut through the outer cortical and cancellous layers of bone. The final thin inner cortical bone can be removed with a 1-mm Kerrison rongeur or diamond burr under continuous irrigation. The floating lamina is then carefully elevated off the dura with the aid of an angled curette to separate adhesions between the ligament and dura (Fig. 10-3). Epidural bleeding may be brisk, especially in patients with highly stenotic canals, but this is usually easily controlled with bipolar electrocautery and topical hemostatic agents. Care should be taken to extend the laminectomy to the medial edges of the pedicle to ensure complete decompression. When possible, avoid removing the spinous processes of C2 and C7 as the nuchal ligaments attach at these sites and compromise of the integrity here may contribute to the development of a kyphotic deformity. Partial laminectomy of the cranial aspect of C7 may allow sparing of that level with adequate decompression of the C6-C7 interspace. Another alternative, which is not covered in this chapter, is to combine laminoplasty and posterior fusion (Fig. 10-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree