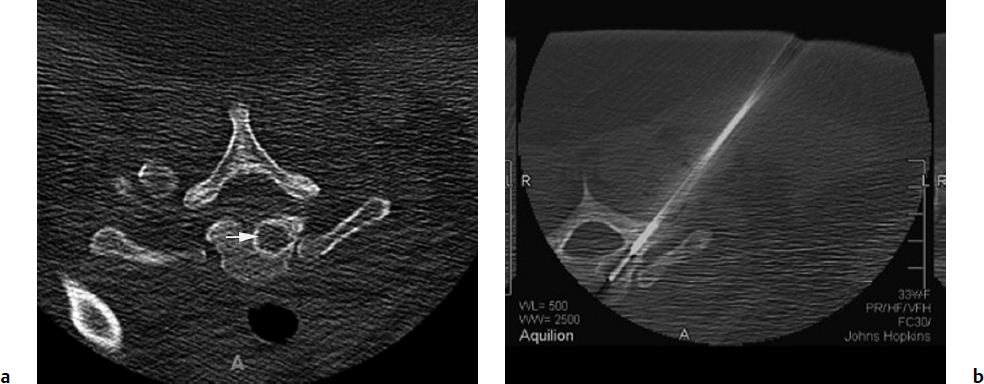
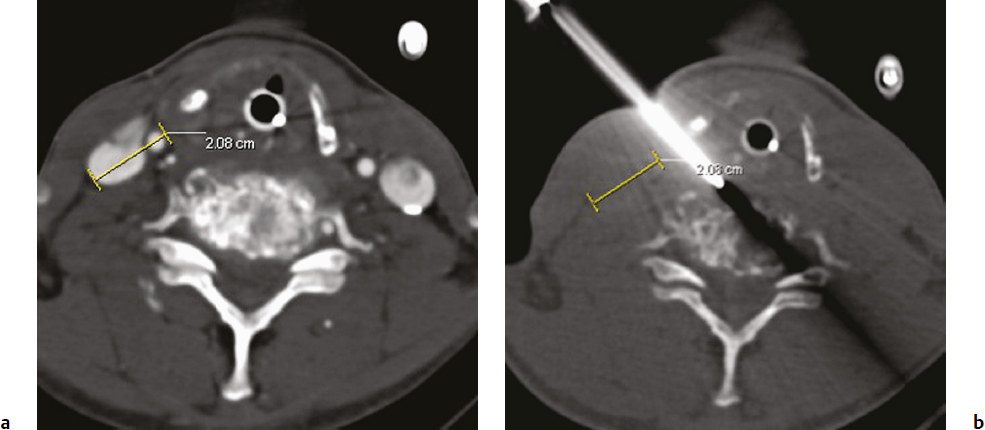
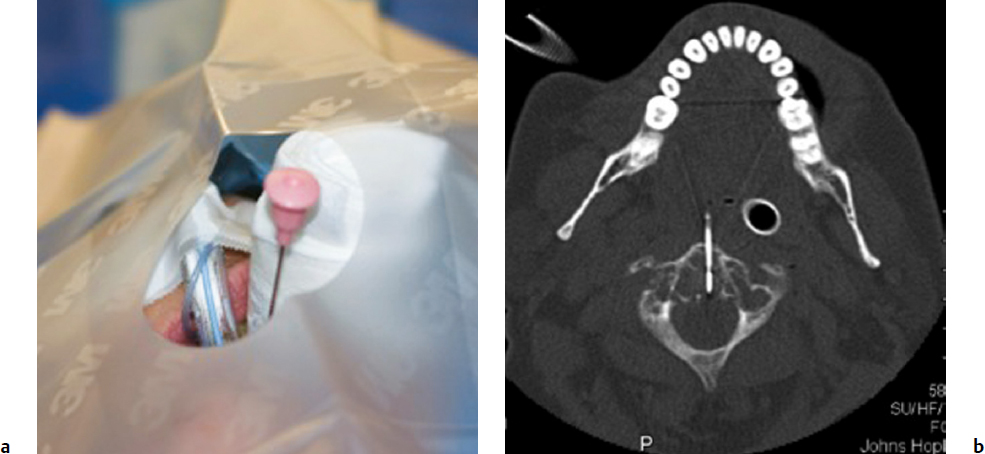
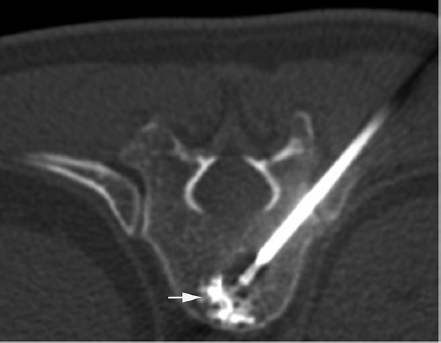
3 Minimally invasive image-guided interventions have undergone a rapid evolution in the last two decades. Most of this evolution has been technologically driven and has included advances in real-time use of high-resolution imaging during interventions; new and improved embolic agents, bone cements, and catheters; and guidewire technology. Percutaneous techniques are being increasingly utilized in both diagnostic and therapeutic interventions of primary spine tumors. This chapter discusses the interventional techniques of spine biopsy, vertebral augmentation, and preoperative embolization, with an emphasis on how they apply to primary tumors of the spine. The biopsy represents the final phase of the local workup for a spinal lesion. If primary spinal tumor is in the differential diagnosis, then the physician doing the biopsy should discuss the plan with the spine surgeon coordinating the patient care to ensure that the biopsy tract will not compromise definitive management. Ideally, the biopsy should be performed in the same center that will provide the definitive treatment, and should involve consultation with the musculoskeletal pathologist to consider any special precautions. Percutaneous image-guided spine biopsy is now commonly performed to diagnose a suspected neoplastic process. Compared with open surgical biopsy, it is less invasive and more cost-effective, with an overall lower risk of complications.1 Determining whether a lesion is benign or malignant and its specific histological type and grade is vital for subsequent management and treatment planning. The decision to perform a spine biopsy should be made after a thorough analysis of risks and benefits. The general risks of percutaneous spine biopsy include bleeding, injury to vascular or neural structures, and infection. In certain tumors, such as chordoma, disease can spread along the needle tract due to tumor implantation. Increasing use of image guidance and coaxial needle technique has decreased the incidence of these complications. Certain complications are specific to the anatomic structures that are in proximity or along the path of needle placement during the biopsy procedure. Pneumothorax can occur during biopsy of thoracic spine or lesions adjacent to the lung or pleura. Stroke due to injury to the carotid or vertebral artery is a concern when performing cervical spine biopsy.2 Most spine biopsies can be performed on an outpatient basis using local anesthesia and moderate sedation. General anesthesia may be necessary in certain situations, such as cervical spine biopsy where patient motion is risky, transoral C2 lesion biopsy where intubation is required to avoid aspiration, and some underlying medical conditions. The patient is generally positioned prone for thoracic or lumbosacral spine biopsy and supine for cervical spine biopsy, except for certain lesions located within the posterior elements for which the prone position may be more appropriate. In certain instances, for example, when a patient has a colostomy or gastrostomy tube and is unable to lie completely prone, biopsy can be performed in the lateral decubitus or semi-prone position. Strict aseptic technique should be used during this procedure to minimize any iatrogenic infections. The imaging modalities available for guidance include X-ray fluoroscopy, computed tomography (CT) fluoroscopy, magnetic resonance imaging (MRI); they can be used singly or in combination. The choice of modality is determined by its availability, the size and location of the suspected lesion, and operator preference. X-ray fluoroscopy has the advantage of being able to visualize and navigate the needle in real time using a two-dimensional projection format. Many simple biopsy procedures can be safely performed using this system. However, it lacks the spatial and soft tissue contrast resolution. I prefer CT fluoroscopy in most cases as it provides excellent spatial and contrast resolution for precise lesion localization and identification of important intervening structures. This enables the operator to select a safe biopsy needle trajectory to access the target lesion. CT fluoroscopy provides a prompt display of continuously updated images that show the exact needle location in relation to the target lesion and important normal structures in the vicinity. This reduces the risk and significantly shortens the procedure time. CT fluoroscopy is extremely helpful in approaching lesions in anatomically difficult areas.3 Magnetic resonance (MR)-guided biopsy procedures can be safely performed in clinical practice. Although CT fluoroscopy currently is the modality of choice, MR guidance provides distinct advantages in certain situations. Most cystic lesions and certain bony lesions such as hemangiomas are better detected on MRI. A major advantage of an MR-guided procedure is the lack of radiation exposure to both patients and physicians, which is especially important in children and pregnant women. Current major limitations of this modality are longer procedure time, higher cost, and limited availability of machine and instruments. However, development of new MRI guidance methods and devices is rapidly evolving. There are several commercial biopsy needle systems available. The selection depends on lesion location and whether the lesion is soft tissue, cystic, or osseous in nature. Aspiration biopsy of a cystic or soft tissue lesion can be performed with 25-, 22-, or 20-gauge needles specially designed to aspirate cellular material. Core biopsies are performed using soft tissue cutting needles (16- or 18-gauge) or trephine and beveled tip bone biopsy needles. We recommend using a coaxial needle technique where only a single guiding needle/cannula pass is made from the skin to the lesion. This technique requires only a single biopsy tract within the soft tissue, thus reducing procedure time and risk of soft tissue injury associated with additional passes. The guiding needle provides access for several biopsy needle passes inside the lesion. This technique also reduces the risk of infiltration of normal tissues with potential tumor cells from the biopsy needle.2 The appropriate biopsy approach depends on lesion size, location, and intervening anatomic structures. Thorough knowledge of the anatomy is essential for planning a safe route of access for needle placement. In the cervical spine, the critical anatomic structures include the vertebral and carotid artery, jugular vein, esophagus, trachea, pharynx and hypopharynx, thyroid gland, segmental nerves, and spinal cord. In the thoracic spine anatomic knowledge of the pleura, lung, aorta, radicular arteries, and neurologic structures is critical. In the lumbar spine, the conus and exiting roots, kidneys, bowel, and major blood vessels must all be considered.4 A posterior approach is generally used for sacral, lumbar, thoracic, and posterior cervical lesions. The patient is placed prone or in the lateral decubitus position. The biopsy needle can enter the vertebral body via a transpedicular (Fig. 3.1) or parapedicular approach. Generally the treating surgeon would prefer the biopsy tract to be as close to the midline as possible and to be marked with a suture or dye. Due to smaller pedicle size, an anterior approach is often used for most cervical spine lesions. The needle is inserted adjacent to the medial border of the sternocleidomastoid muscle and advanced between the carotid sheath and neck airways (Fig. 3.2). Special attention should be paid to avoid the esophagus and hypopharynx. This procedure should be done with CT fluoroscopy guidance, and intermittent CT images should be obtained to check the needle trajectory. Fig. 3.1a,b Posterior approach. (a) Computed tomography (CT) scan of a patient in the prone position shows a well-defined lytic lesion (arrow) in the T1 vertebral body. (b) CT-fluoroscopy–guided transpedicular biopsy needle placement from the posterior approach inside the lesion. The transoral approach can be very useful for accessing C2 vertebral body lesions. It can also be used to access lesions at adjacent levels including C1 and C3. This approach requires general anesthesia, as intubation is necessary to avoid any aspiration (Fig. 3.3). Prophylactic antibiotics should be used with this approach, as it is difficult to create a sterile field along the posterior pharyngeal wall though which the biopsy needle is inserted. This is a relatively safe approach, as no important structures lie between the posterior pharyngeal wall and the bone.5 Fig. 3.3a,b Transoral approach. (a) Close-up view of the intubation tube and transoral biopsy needle inserted through an open mouth. Note the gauze roll used to keep the mouth open. (b) CT-fluoroscopy image shows the biopsy needle tip inside the lesion. Direct compression is applied at the puncture site for about 5 to 10 minutes to achieve hemostasis. This is followed by placement of sterile dressing on the puncture site and adjacent skin. The patient should be kept in the recovery area for 1 to 2 hours to watch for any bleeding; to assess pain, weakness, and dizziness; and to monitor the general vital signs. The patient is discharged with a medication prescription and a contact number to reach a physician if any complications arise. Vertebral augmentation is a minimally invasive procedure primarily utilized for treating back pain associated with a vertebral compression fractures. This procedure was first introduced as a technique for the treatment of symptomatic vertebral hemangioma in 1987.6 Since that time, it has become a valuable and frequently used therapeutic option in the management of back pain caused by osteoporotic and pathological fractures. The fundamental goal of a vertebral augmentation procedure is to stabilize and improve the compressive strength of the vertebral body through the safe injection of stabilizing material. This can be achieved by both vertebroplasty and kyphoplasty. Vertebroplasty involves the injection of special bone cement inside the fractured vertebra using a needle under image guidance, generally X-ray or CT-fluoroscopy. There are several bone cements available, but the most commonly used is acrylic polymer polymethylmethacrylate (PMMA). Kyphoplasty, in comparison with vertebroplasty, involves an additional step of creating a cavity inside the diseased vertebral body by inflating a balloon followed by injection of the bone cement in the cavity. Malignant lesions can spread to the spinal column by hematogenous, lymphatic, perineural, or direct extension.7 Pain arises from cortical erosion, fracture, or impingement on the nerve roots or spinal cord. There are various treatment options that depend on the underlying nature of the disease and include surgery, medical therapy, radiation therapy, or a combination of them. Vertebral augmentation is now playing an increasing role in this patient population. The decision regarding therapy should be made by a multidisciplinary team taking into consideration the nature and extent of the disease, the medical condition of the patient, the response to prior therapy, and the patient’s life expectancy. The goal of vertebral augmentation in pathological fractures is to improve pain and structural stability of the bone. Radiation therapy is effective in many cases, but if there is spinal instability present, pain relief will not occur with radiation alone as there is no immediate bone strengthening.8 Vertebral augmentation can be a good therapeutic option by itself or can be combined with radiation and surgical therapy. Absolute contraindications for vertebral augmentation include ongoing local or systemic infection, compressive myelopathy secondary to retropulsed bone fragment or tumor, and any uncorrectable coagulopathy. Relative contraindications unique to pathological fractures depend on the operator expertise and the tools available. Severely compressed vertebral bodies with epidural tumor extension are technically difficult to treat and may be made worse with injection of PMMA. Some of these cases can be treated by an experienced operator using a plasma-mediated tumor ablation system to first create a cavity within the tumor, followed by judicious PMMA cement injection.9 The use of CT-fluoroscopy with excellent spatial and contrast resolution can be very useful in such cases, as they require precise needle placement and close monitoring of PMMA cement injection. Cross-sectional imaging, including MRI and CT, is extremely useful for identifying the location and extent of the disease. It also provides useful information about any canal or neural compromise. CT in particular is very helpful in demonstrating osseous destruction that can be a potential route of cement extravasation and for assessing transpedicular access. A variety of disposable vertebroplasty and kyphoplasty needles are available. Needles range in sizes from 8 to 13 gauge. Smaller size 15-gauge needles are also available and more suitable for cervical spine. Several PMMA-based stabilizing agents with different characteristics are commercially available that can be used for vertebral augmentation. The basic principles and techniques of safe needle placement described earlier in the spine biopsy section also apply to vertebral augmentation. A posterolateral approach is generally preferred for lumbar and thoracic spine lesions. Special attention should be paid to avoid breaking medial and inferior borders of the pedicles. Breach of these cortical walls can result in needle entry into the spinal canal or neural foramen, with potential injury to the spinal cord or to nerves. For cervical spine lesions, anterolateral approach is generally more suitable. For lesions within the C2 vertebral body, a transoral route may be more favorable. The needle is advanced using continuous or intermittent fluoroscopy until the tip is placed in desired location, generally in the anterior part of the tumor. Most injection devices that are available are self-contained systems, with a reservoir into which the PMMA cement is filled. This device is then connected with the needle and the PMMA can be injected in a controlled fashion by rotation or trigger mechanism (Fig. 3.4). PMMA should be injected in small boluses with close attention to any leakage beyond the confines of the vertebral body, especially into pulmonary veins, epidural veins, the central canal, or the neural foramen. A small leak through end-plate fractures may be acceptable, but a large amount of leakage within the disk space may increase the future risk of adjacent fracture.10 If the PMMA preferentially flows to undesirable areas, the needle can be repositioned, and the material already injected is allowed to harden. A coaxial technique is very helpful in such instances, as the cement-injecting needle can be taken out while keeping the access needle in place. This allows the cement already injected in the body to harden faster, as it is exposed to a body temperature that is higher than room temperature. Injection is then resumed in few minutes, with expectation of PMMA cement now filling in the desirable area. If the PMMA continues to flow in undesirable areas, further injection should be stopped.
Interventional Options for Primary Tumors of the Spine

 Introduction
Introduction
 Image-Guided Spine Biopsy
Image-Guided Spine Biopsy
 Patient Preparation
Patient Preparation
 Image Guidance
Image Guidance
 Biopsy Needle Systems and Technique
Biopsy Needle Systems and Technique
 Biopsy Approaches for Spine Tumors
Biopsy Approaches for Spine Tumors
Posterior Approach
Anterior Approach
Transoral Approach
Postprocedure Care
 Vertebral Augmentation for Spine Tumors and Pathological Fractures
Vertebral Augmentation for Spine Tumors and Pathological Fractures
Patient Selection
Preprocedural Imaging
Tools, Technique, and Special Considerations
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree