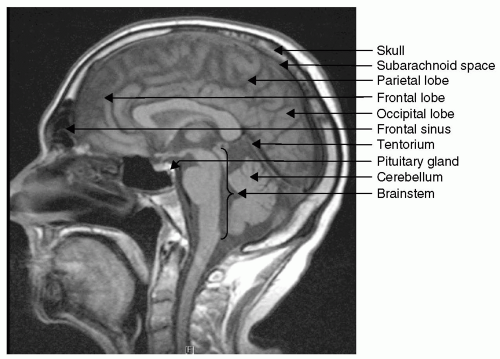
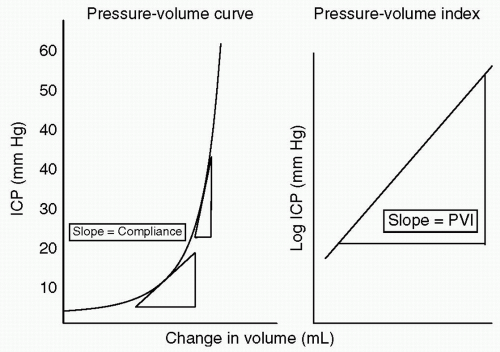
how the intracranial contents are able to compensate for changes in volume. When compliance is reduced, small changes in volume lead to large changes in ICP. It is important to understand this rightward shift of the pressure-volume index (PVI), (see Fig. 2) because even when ICP is normal, very small changes in volume can have disastrous effects by causing herniation through a rapid increase in ICP.
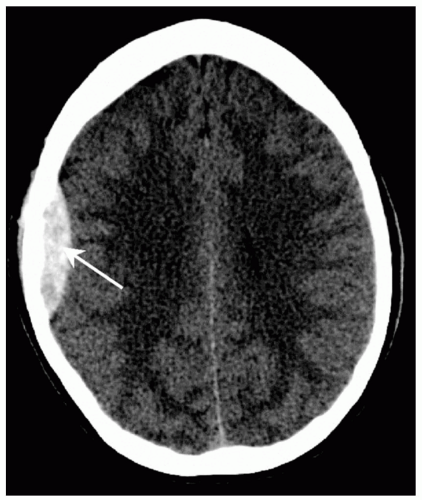
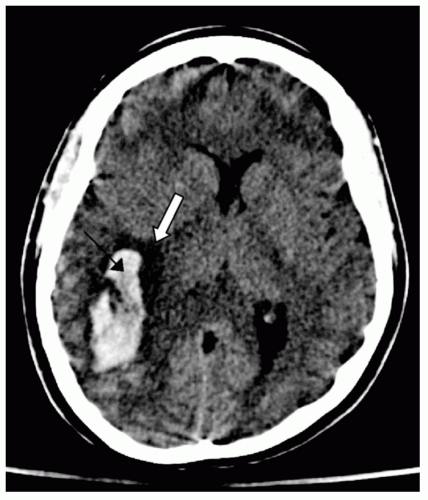
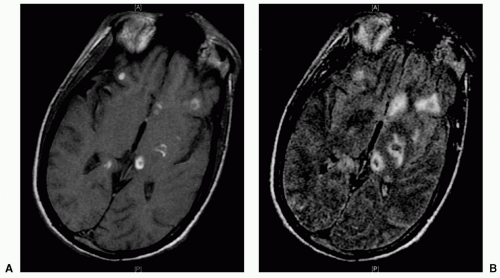
coalesced mass in an ICH on CT scan. Contusions are often found in the frontal and temporal lobes because this tissue “glides” over the underlying rough bony surface of the skull base during impact and acceleration/deceleration of the head (see Fig. 6). The brain may also impact the inner table of the skull across from the area of impact, thereby causing a contusion or hemorrhage. This is known as a coup-contre-coup lesion. ICH may also occur with penetrating injuries (e.g., stab wounds or gun shot wounds, see subsequent text). Finally, ICH may develop or progress ≥24 hours after injury (delayed traumatic ICH) and therefore, patients at risk require careful follow-up and repeat imaging.14,15
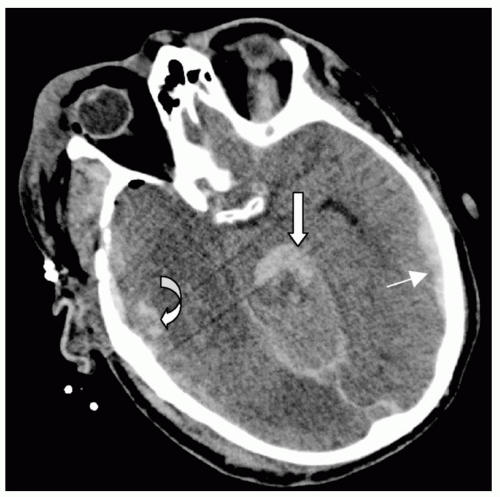
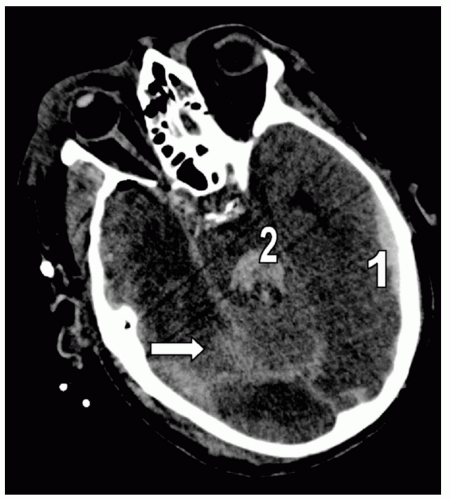 Figure 7 Subarachnoid hemorrhage (SAH). Different detail from the patient in Fig. 5 demonstrating extensive right posterior SAH (arrow). Note the accompanying left temporal subdural hematoma (1) and fourth ventricle blood (2). |
before administration of sedatives and/or paralytics. A GCS of ≤8 indicates coma and airway intubation, and ventilation should be considered early. If necessary, an emergency surgical airway (cricothyroidotomy, tracheostomy) may be required.
TABLE 1 GLASGOW COMA SCALE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 2 CLASSIFICATION OF TRAUMATIC BRAIN INJURY (TBI) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree