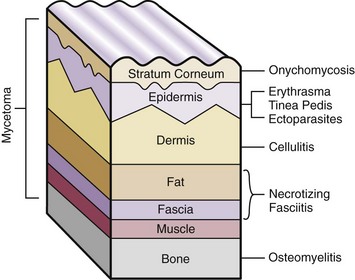
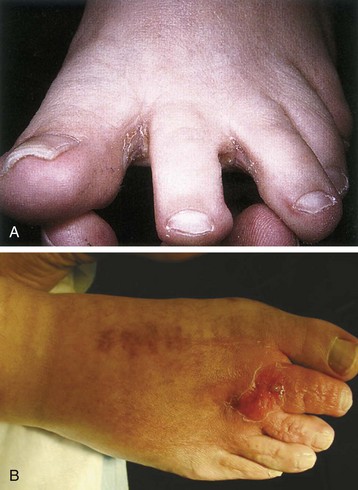
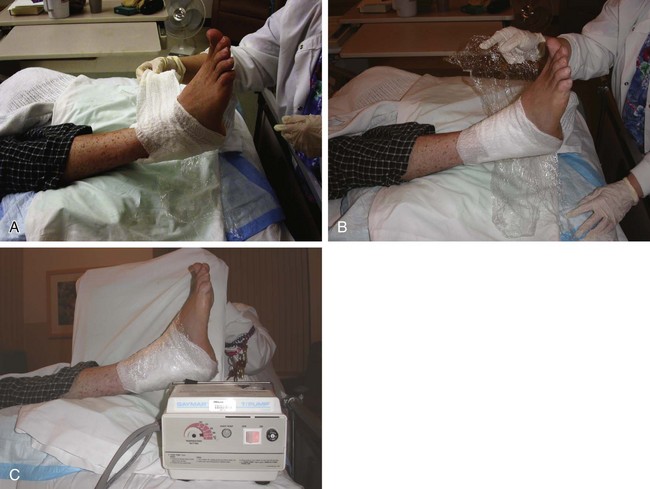
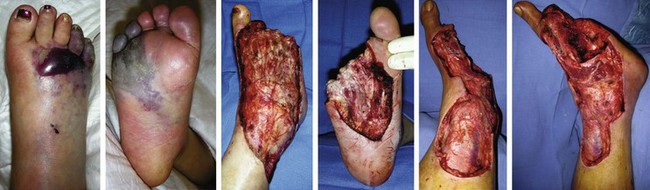
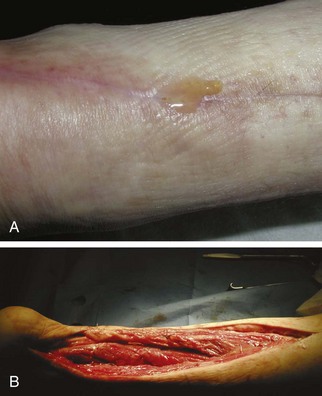
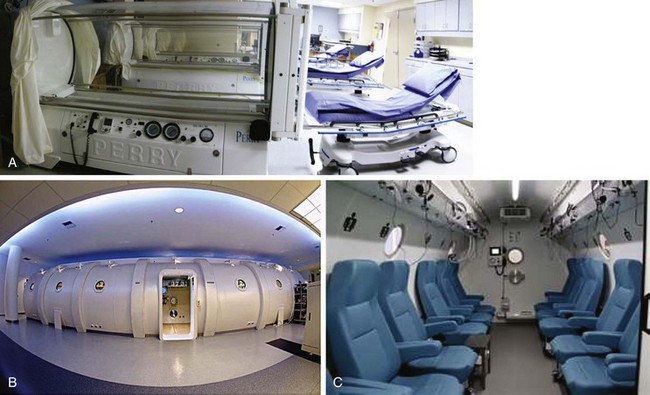
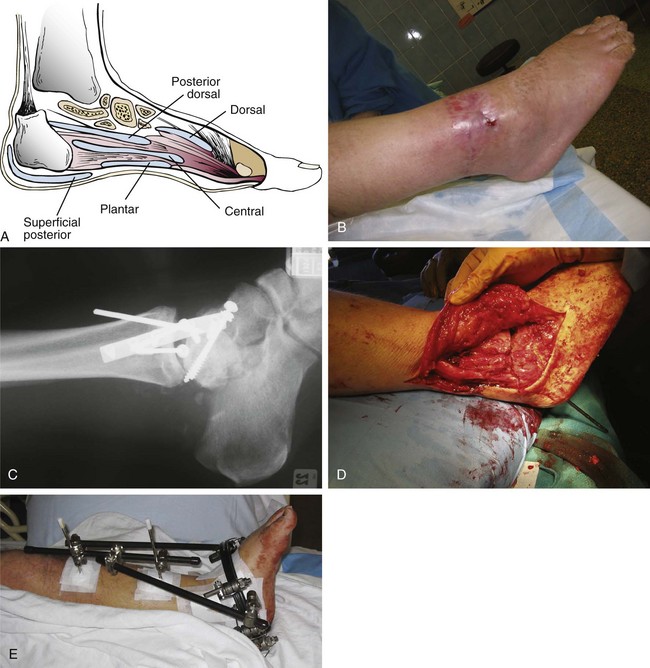
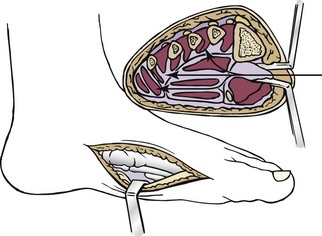
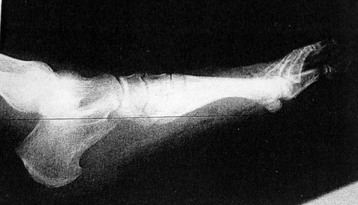
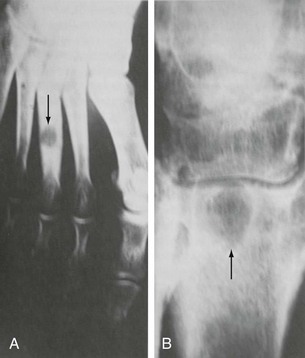
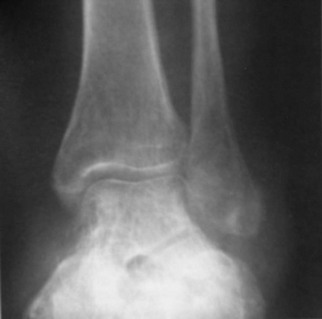
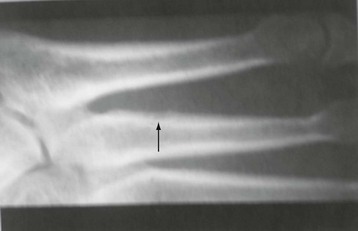
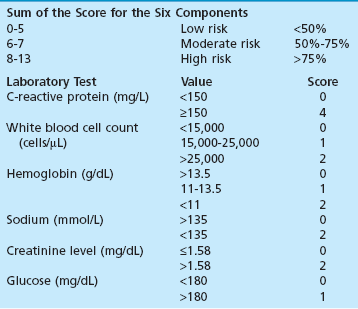
Chapter 15 Natural Penicillin (Penicillin G) Penicillinase-Resistant Penicillin (Methicillin, Nafcillin, Dicloxacillin) Aminopenicillins (Ampicillin, Amoxicillin) Antipseudomonal Penicillin (Ticarcillin) Aminoglycosides (Gentamicin, Tobramycin, Amikacin) First-Generation Cephalosporins (Cephalexin, Cefazolin) Second-Generation Cephalosporins (Cefotetan, Cefoxitin) Third-Generation Cephalosporins (Cefotaxime, Ceftriaxone, Ceftizoxime, Ceftazidime) Second-Generation Fluoroquinolones (Ciprofloxacin, Ofloxacin) Third-Generation Fluoroquinolones (Levofloxacin, Sparfloxacin) Fourth-Generation Fluoroquinolones (Trovafloxacin, Moxifloxacin, Gatifloxacin) Infections are classified to assist with treatment selection. Classification is by host type, organism, or anatomic location. Host factors are the most significant. Hosts can be classified as normal or compromised. A compromised host changes both the differential diagnosis and the treatment of a foot infection. Compromised hosts include patients with systemic illness, poor nutritional state, peripheral vascular disease, trauma, and peripheral neuropathy. Diabetes is the most common systemic host factor in a patient with foot infection. Diabetic foot infections are considered in Chapter 27. Soft tissue infections often present secondary to trauma to the protective stratum corneum of the epidermis (Fig. 15-1). Surgery, injury, maceration, intravenous (IV) drug abuse, eczema, and constitutionally derived skin breaks can provide a portal of entry for the pathologic organism. Blisters, ulcers, and open wounds are often colonized with bacteria. In a compromised host, these bacteria can spread, resulting in a localized, and ultimately systemic, infection. More virulent organisms can use a portal to spread within a normal host. The offending organism can often be deduced from the point of entry and host status. For example, postoperative wound infections in a normal host are often group A streptococci.5 Breaks in the epidermis are not necessary for soft tissue to become infected. Some virulent organisms possess toxins that weaken the skin’s defenses. These organisms are able to attack healthy hosts without an entry portal.34 Staphylococcus aureus is the primary pathogen to penetrate intact skin. It manifests as cellulitis or an abscess. The scaly lesions vary from red to brown, are well defined, and have irregular borders (Fig. 15-2A). The lesions are most commonly asymptomatic but can be pruritic. The lesions can become fissured and macerated, creating breaks in the protective stratum corneum. These openings can result in more extensive infections. Erythrasma is commonly confused with psoriasis, dermatophytosis, and candidiasis. A Wood lamp examination can help distinguish erythrasma because the colonies glow coral-red. Erythromycin (250 mg four times daily for 14 days) is the treatment of choice.50 Cellulitis is the acute inflammatory response to a pathogen, often bacterial. Aerobic gram-positive cocci, S. aureus and streptococci, are the most commonly involved pathogens. Cellulitis is characterized by superficial swelling, pain, erythema, and localized warmth (Fig. 15-2B). Proximal lymphadenopathy is not uncommon. Erythema streaking along the course of lymph drainage (lymphangitis) is common with streptococcal infections, while localized pus-producing lesions are staphyloccal.33 Cellulitis is limited to the dermis and subcutaneous tissues. The treatment of cellulitis uses empiric antibiotics directed against Staphylococcus and Streptococcus species.5 The affected limb should be elevated to help control swelling. Moist heat is applied to the affected area using a Koch-Mason dressing. This dressing consists of a layer of moist gauze covered with an occlusive barrier. A warming blanket is wrapped over the barrier (Fig. 15-3). The rise of methicillin-resistant S. aureus (MRSA) has complicated the selection of antibiotics. This organism is resistant to the β-lactam antibiotics, including the penicillins and cephalosporins. MRSA is now found in hospital-acquired and community-acquired infections. The risk factors for hospital-acquired MRSA are recent hospitalization, recent surgery, dialysis, residence in a long-term care facility, diabetes, and chronic or repeated courses of antibiotics.33,118 Fifty-seven percent of nosocomial isolates of S. aureus are resistant to methicillin.110 The risk factors for community-acquired MRSA are young age, socially disadvantaged environment, minority ethnicity, member of the armed forces, an athlete, and primary skin infections.33,34 MRSA transmission during team sports has been documented and should be considered in the differential diagnosis of clusters of skin abscesses in athletes.57 A community’s bacterial spectrum can assist in determining the risk of community-acquired MRSA in the general population. For patients with mild cellulitis and no risk factors for MRSA, treatment involves oral first-generation cephalosporins, such as cephalexin 50 mg/kg per day (2 g divided into 4 doses for adults) divided every 6 hours. For more severe cases, systemic involvement, or a compromised host, parenteral antibiotics are first-line agents. Cefazolin 80 mg/kg per day (4 g for adults) IV divided four times daily is often the primary agent. An aminoglycoside, gentamicin 5 mg/kg every day, should be added for diabetic patients and IV drug abusers to provide a broader coverage for possible gram-negative species.5 Alternative medications for infections at risk for both gram-positive and gram-negative organisms are β-lactams/β-lactamase inhibitory combinations, moxifloxacin, tigecycline, or trimethoprim-sulfamethoxazole.33 For patients with risk factors for MRSA infections, alternatives to β-lactams should be first-line treatments. Initial treatment of MRSA infections with β-lactams can exacerbate the infection or compromise outcomes by delaying appropriate treatment. For mild cases, clindamycin, a fluoroquinolone (moxifloxacin has extended gram-positive activity), tetracycline, or trimethoprim are acceptable choices.33 Selection of an antibiotic depends on resistance spectrums of the community and patient factors. For severe infections with suspected MRSA or failure of a β-lactam, parenteral treatment with linezolid, daptomycin, tigecycline, or vancomycin is appropriate. Because of concerns over the cost and the possibility of resistance to these agents, these antibiotics should be reserved for severe infections with a high clinical suspicion of MRSA. Necrotizing fasciitis is a rapidly destructive infection of soft tissues with a high mortality rate. Early diagnosis is the key to effective management. This infection spreads along the fascial planes and is associated with thrombosis of the cutaneous microcirculation. It generally affects skin, subcutaneous tissues, and fascia. It is often associated with streptococcal species; however, the majority of cases are polymicrobial.23,35,120 A portal of entry is found in only half of all patients.120 Risk factors include diabetes, obesity, peripheral vascular disease, intravenous drug use, alcohol abuse, malnutrition, smoking, corticosteroid therapy, immune suppression, cancer, and age.13 Nonsteroidal antiinflammatory drugs (NSAIDs) can delay the diagnosis of necrotizing fasciitis by reducing the symptoms but have not been shown to be a risk factor.10 The clinician should maintain a high clinical suspicion of necrotizing fasciitis. Because of the limited clinical signs, an initial diagnosis of cellulitis is common. The definitive signs of necrotizing fasciitis are hemorrhagic bullae, skin necrosis, fluctuance, crepitus, and neurologic deficits. These definitive signs are often not found until late in the disease progression.120 Delay in treatment can significantly increase the risk of mortality. Patients with a risk factor, pain out of proportion to clinical findings, or subcutaneous air on radiographs should be considered at risk for necrotizing fasciitis. The threshold for surgical debridement should be low in these patients. Failure of intravenous antibiotics in the treatment of cellulitis points to an early presentation of necrotizing fasciitis. Laboratory data may be useful in identifying high-risk patients presenting with cellulitis but should never delay surgical treatment of patients with clinical presentation consistent with necrotizing fasciitis. Table 15-1 lists the components of the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC). Patients with a score of 6 to 7 should be considered moderate risk, and greater than 7 should be considered high risk.121 Interventions to correct laboratory disturbances can reduce the accuracy of the score, but an increasing score despite antibiotics may indicate the presence of necrotizing fasciitis. Table 15-1 LRINEC, Laboratory Risk Indicator for Necrotizing Fasciitis. Modified from Wong C, Khin, L, Heng K, et al: The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections, Crit Care Med 32:1535–1541, 2004. The risk factors for necrotizing fasciitis include smoking, diabetes, IV drug abuse, and peripheral vascular disease.35,119,120 The lower extremity is the most commonly affected region.23 The foot is the second most common site in the lower extremity.35 Patients rapidly deteriorate. Leukocytosis, weeping blisters, blue skin discoloration, sensory deficits, systemic sepsis, shock, and mental status changes are late manifestations.23,119 Figure 15-4 shows the clinical appearance of necrotizing fasciitis. Treatment of necrotizing fasciitis involves early surgical debridement and broad-spectrum antibiotics. Because of the thrombosis of microcirculation, antibiotics alone are ineffective. In suspicious cases, a limited biopsy is often warranted. A 2-cm incision is made under local anesthesia. The surgeon bluntly dissects down to the deep fascia. Biopsy specimens are sent for cultures and pathologic examination. A lack of bleeding, dishwater-colored drainage, or minimal resistance to finger dissection along the fascial plane are signs of necrotizing fasciitis.23,120 An aggressive surgical debridement should be extended until the skin and subcutaneous tissue appear viable and firmly adherent to the underlying fascia (see Fig. 15-4). All necrotic skin, subcutaneous tissue, fascia, and muscle should be removed (Fig. 15-5). Repeat debridement should be carried out at 48-hour intervals.35,120 Hydrogel or negative-pressure therapy dressings are used to promote granulation tissue.13 Medical treatment with antibiotics starts empirically with a combination of a penicillin, an aminoglycoside, and clindamycin. Clindamycin and linezolid inhibit M protein and exotoxin production, facilitating phagocytosis and suppressing the production of tumor necrosis factor-α.13 Culture results are used to modify the antibiotic regimen. Coagulopathy, cardiopulmonary collapse, and acute renal failure are seen in the more advanced stages of necrotizing fasciitis. Blood component therapy, dialysis, and cardiopulmonary support should be used aggressively when indicated. A patient’s metabolic requirements are doubled during the treatment period. Supplemental feedings or total parental nutrition should be considered. Hyperbaric oxygen is used as an adjunctive treatment at some medical centers.13,23 Hyperbaric chambers come in single- and multiple-place systems (Fig. 15-6). Studies have demonstrated a beneficial trend, but there is no conclusive evidence for the effectiveness of hyperbaric oxygen. Prompt surgical debridement continues to be the most important factor in reducing mortality.23,35,119,120 An abscess is a collection of purulent material and infection in a closed space. In the foot, the infection is usually an extension of a subcutaneous infection or penetrating wound. Often, abscesses involve the deep spaces of the foot (Fig. 15-7A). With a deep-space infection, the patient presents with pain and swelling along the instep. Examination demonstrates a loss of the longitudinal arch. Patients often become bedridden because of the pain of weight bearing. Loeffler and Ballard70 described five plantar spaces (Table 15-2). Table 15-2 Plantar Fascial Spaces of the Foot The infection is often polymicrobial. Without treatment, the infection will spread along the flexor tendons, extending into the deep compartment of the leg (Fig. 15-7B-E). Surgical debridement is essential in treating the abscess. Appropriate antibiotics are based on intraoperative cultures. If the infection appears to be localized more medially, a medial single-incision fasciotomy can be used to expose the infected compartment. The majority of deep abscesses of the foot occur in the central plantar space. If the infection appears to localize more dorsally in the forefoot, a two-incision fasciotomy approach allows ready exposure to the more dorsal spaces. The surgical approach described by Loeffler and Ballard is as follows.70 1. The incision begins posterior to the medial malleolus and ends between the first and second metatarsal heads. The incision runs along the midline of the plantar surface of the foot. 2. The plantar aponeurosis is divided. This opens the superficial plantar space and the space located just dorsal to the aponeurosis. 3. The dissection continues through the interval between the abductor hallucis and flexor digitorum brevis. This exposes the large space between the flexor digitorum brevis and the quadratus plantae. The plantar arteries and nerves run in this space. 4. The abductor hallucis and flexor digitorum brevis are detached from the calcaneus and are retracted anteriorly. This exposes the quadratus plantae muscle. The flexor hallucis longus tendon is separated from the quadratus plantae, exposing the compartment dorsal to the quadratus plantae. 5. This dissection is continued distally, keeping the plantar nerves visualized. The final compartment is entered deep to the adductor hallucis muscle. 6. The wound is irrigated and left open. Delayed primary closure can usually be achieved in 48 to 72 hours. The medial single-incision approach is as follows (Fig. 15-8). 1. The incision runs the length of the first metatarsal just along its inferior border. 2. Blunt dissection creates a plane between the first metatarsal and the abductor hallucis muscle. 3. Blunt finger dissection continues into the space dorsal to the quadratus plantae more proximally. 4. Blunt finger dissection is then directed dorsal to the adductor hallucis into the space dorsal to the adductor hallucis. Sharp dissection should be avoided because of the presence of the plantar arteries and nerves in this space. 5. Blunt dissection then separates the quadratus plantae and the flexor digitorum brevis to enter this space. 1. Two longitudinal incisions are made directly above the second and fourth metatarsals. The incision runs from the base of metatarsal to the metatarsal neck. 2. Using a blunt instrument, spread longitudinally directly down to bone. 3. The intermetatarsal spaces are entered by blunt dissection adjacent to the metatarsals. 4. The dissection can be extended down to the quadratus plantae muscle. It is difficult to reach the medial and lateral compartments from these incisions. Gas gangrene can follow trauma or surgical procedures. Clostridium species and mixed aerobic and anaerobic infections can produce gas in the soft tissues. The mixed infections show abundant gas on radiographic or magnetic resonance imaging (MRI) studies (Fig. 15-9). Clostridial infections are characterized by muscle necrosis on MRI. Patients with gas gangrene are acutely sick, but they often do not have fever. The affected area is painful, with overlying bullae. These bullae are filled with sweet-smelling fluid if they are infected with clostridia. With mixed infections, the bullae are typically filled with foul-smelling fluid. Clostridium septicum is seen in nontraumatic clostridial infection associated with malignancy.107 An infected joint can develop from an adjacent infection, direct traumatic inoculation, or hematogenous spread. Septic arthritis is recognized clinically by the abrupt appearance of redness, swelling, and tenderness about the joint.43 Fever is not a prerequisite for the diagnosis of septic arthritis.77 Infection should be a primary consideration in any acute monoarticular arthritis, followed by crystal arthropathy, Reiter syndrome, or a neuropathic joint. Adjacent soft tissue swelling and increased joint fluid can be detected on imaging studies. The diagnosis is made by joint aspiration before antibiotic treatment. The joints most commonly affected are the knee, hip, and ankle.58 Multiple joints are involved in 20% of cases.77 Treatment of suspected septic arthritis begins with aspiration of the joint. The fluid obtained is analyzed for color, Gram stain, cultures, glucose, protein, crystals, and cell count with differential. Aspiration through an area of cellulitis should be avoided.89 Other studies to consider include peripheral blood cell count, C-reactive protein, erythrocyte sedimentation rate, uric acid, and blood cultures. Joint fluid analysis generally shows leukocyte counts greater than 100,000/mm3, 90% polymorphonuclear leukocytes, elevated protein, and glucose level less than that of serum.76 The standard definition of a positive joint aspiration is a leukocyte count greater than 50,000.43,67,89 Using that standard, the sensitivity of the examination is only 64%.67 Any leukocyte count greater than 10,000 cells/mm3 should be considered suspicious of a joint infection and the patient closely monitored. Gram staining has a sensitivity of 50%.43 Cultures are generally positive unless the organism is Neisseria gonorrhoeae or the patient received antibiotics before the aspiration. Table 15-3 provides a guideline for interpreting laboratory studies. Table 15-3 PMN, polymorphonucleocyte; WBC, white blood cell. *Degenerative joint disease, neuropathy, systemic lupus erythematosus, rheumatic fever. †Gout, pseudogout, systemic lupus erythematosus, psoriasis, Reiter disease, rheumatoid arthritis. The joint fluid should also be examined under polarized microscopy for crystals. Both gout and pseudogout can mimic a septic joint. Cell count alone cannot distinguish gout from a septic joint.77 For patients with no mechanism for direct inoculation of the joint, blood cultures should be obtained. Blood cultures will return the causative agent in approximately 30% of cases. Diabetes mellitus, joint prosthesis, inflammatory arthritis (rheumatoid), osteoarthritis, crystal arthropathy, cutaneous ulcers, and alcoholism predispose a patient to septic arthritis.77 Antitumor necrosis factor therapy doubles the risk of septic arthritis.42 S. aureus is the most common organism in septic joints. In children younger than 2 months, group B streptococci are the most likely pathogens. Neisseria gonorrhoeae is a common pathogen in the sexually active young adult. In these patients, an enriched agar with high carbon dioxide culture should be included in the joint aspirate studies. Puncture wounds can inoculate the joint with anaerobic and gram-negative species. Drainage of purulent material from the joint is essential in the treatment of septic arthritis. This may be by open surgical procedure or aspiration. The method chosen is controversial. There is little evidence to support open surgical drainage of an infected joint in the foot.73 Daily aspiration of the affected joint is often sufficient. Indications for surgical intervention include failure to clinically respond within 48 hours of therapy, persistence of pyarthrosis beyond 48 hours, inaccessibility of the joint for aspiration, viscous material that defies attempts at aspiration, and radiographic changes affecting bone.55 Many surgeons do not feel they can adequately drain the joints in the foot by aspiration alone and prefer open drainage for all septic joints in the foot. Surgical decompression requires adequate exposure of the joint, a synovectomy, and a thorough irrigation with saline. The joint is allowed to continue to drain using suction or a Penrose drain for 24 to 48 hours. The foot should be splinted to allow relative rest and for patient comfort. If the symptoms have not resolved, repeat irrigation and debridement should be undertaken at 48 hours. The use of antibiotics in the irrigation has not been shown to have superior results to saline alone.43 Broad-spectrum empiric antibiotics should be administered parenterally. Nafcillin combined with third-generation cephalosporins will cover most infections. For patients with a penicillin allergy, levofloxacin is an accepted substitute. Patients at risk for MRSA (nursing home residents, recent inpatients, or those who live in areas where community-acquired MRSA is higher than 10%) should be covered by an appropriate antibiotic.77 The choice of antibiotic is adjusted depending on the pathogen isolated from the aspirate. Therapy should continue for 4 weeks.89 Osteomyelitis is the infection of bone. It is characterized by acute inflammation, vascular engorgement, edema, cellular infiltration, and abscess formation. The infective agents can be bacteria, fungi, or mycobacteria. The source of the infection can be hematogenous, an adjacent infection, or direct inoculation by trauma or surgery. The infection can be acute or chronic. The clinical presentation varies based on the organism, location, and host factors. A conclusive diagnosis is made with a bone biopsy. The diagnosis of osteomyelitis is clinically important because of the length of therapy required for the treatment of bone infections and the necessity for surgical debridement. Sickle cell anemia, chronic granulomatous disease, and diabetes mellitus predispose a patient to develop osteomyelitis.71 Hematogenous infections are most common in children and can affect any of the bones of the foot. The calcaneus and talus are the most commonly involved sites in the foot, with 7% and 4% of all hematogenous infections, respectively.6 S. aureus is the usual pathogen. A Brodie abscess is a particular form of chronic osteomyelitis found in the lower portion of the tibia, talus, or forefoot.6,113 These abscesses have a sclerotic wall and contain purulent material. A Brodie abscess typically has no systemic symptoms, and laboratory studies are often normal. Treatment of a Brodie abscess is debridement and antibiotics (Fig. 15-10). The clinical presentation of acute osteomyelitis includes fever, local pain, swelling, and tenderness. Laboratory studies may show an elevated white blood cell count, erythrocyte sedimentation rate, and C-reactive protein. In compromised hosts and in patients with involvement of the small bones of the foot, the laboratory studies may not be abnormal. Blood cultures are positive in half of patients.55 Cultures obtained from sinus tracts are often unreliable. Identification of the pathogen requires bone aspiration or biopsy. Bone biopsy and culture has a sensitivity of 95% and specificity of 99% if taken before antibiotics are administered.87 Radiographic changes include osteolysis, periosteal reaction, cortical erosion, and formation of sequestra and involucrum18 (Figs. 15-11 and 15-12). These changes take 10 to 14 days to appear. The early signs of soft tissue swelling, periosteal thickening and elevation, and osteopenia are subtle and are often missed.71 Bone mineral loss of 30% is required for radiographic change to be visible. The radiographic findings resemble malignancy, fractures, and neuropathic conditions. Because of these problems, radiographs have a sensitivity of 43% to 75% and specificity of 75% to 83%. During treatment, radiographic findings often lag behind clinical response. MRI can detect changes in the bone earlier than plain films can. Osteomyelitis is identified by alteration of the bone marrow signal that results in a loss of the normal fatty signal on T1 and the enhancement of edema on T2 images. MRI can also demonstrate secondary signs, such as cellulitis, fluid collection, cortical interruption, and sinus tract formation. The presence of secondary signs can increase the sensitivity of a scan.85 The scan provides precise anatomic details showing adjacent abscess formation, sinus tracts, and the extent of osseous involvement. MRI has a sensitivity of 77% to 100% and specificity of 80% to 100%.84 Gadolinium enhancement can improve the detection of adjacent soft tissue disease.84,86 The use of contrast is particularly helpful in the diabetic foot and postsurgery patient. The presence of surgical implants reduces the effectiveness of MRI. Neuropathic disease, altered weight bearing, and surgery can cause marrow changes consistent with osteomyelitis. Fast spin-echo short T1 inversion recovery (FSE STIR) images are useful for initial screening. Their high sensitivity and rapidity of scan can detect small marrow changes. Patients with positive scans can then undergo additional imaging by using gadolinium-enhanced fat-suppressed T1-weighted sequences. The increased specificity of these examinations helps to eliminate false positives. In cases with discordant marrow signs, secondary signs can be used to determine the presence of osteomyelitis. The secondary signs with highest positive predictive value are cortical interruption, a cutaneous ulcer, and a sinus tract.85 Radionuclide bone scans are extensively used in diagnosing osteomyelitis. The three-phase bone scan changes take only 2 days to appear.49 Osteomyelitis is characterized by accumulation of the tracer in all three phases of the scan. This relatively inexpensive test has a very low false-negative rate. Fracture, neuropathic joints, trauma, surgery, and hyperemia can result in positive test results for three-phase bone scan. The sensitivity of the study ranges from 69% to 100% and specificity from 38% to 82%.18 Three-phase bone scans combined with labeled white blood cells increases the specificity. This combination improves the sensitivity to 73% to 100% and specificity to 55% to 100%.117 The test is complicated and requires an additional hospital day to perform. The combined tests are generally more expensive than MRI. A recent development in imaging for musculoskeletal infections is 18F-fluorodeoxyglucose positron emission tomography (PET). PET evaluates cellular glucose metabolism to identify increased use by activated neutrophils and macrophages. The sensitivity is 91% to 100%, and specificity is 88% to 91%.22,29 An advantage of PET is the ability to differentiate between Charcot lesions and osteomyelitis.53 PET is particularly useful around metal implants, which limit the usefulness of MRI. The studies are limited to facilities with a PET scanner and are expensive. Elevated blood glucose can result in false-negative results. PET should be considered for differentiating Charcot lesions and osteomyelitis or for detecting osteomyelitis near metallic implants (see Chapter 3). The treatment of osteomyelitis depends upon surgical debridement. Antibiotics are less effective in acidic, anaerobic, hypercapnic, and poorly perfused regions. Excision of necrotic bone, removal of purulent material, and elimination of any dead space is essential to the success of antibiotic treatment. The surgical debridement is more important than serum levels of antibiotics or duration of treatment.72 Care should be taken during the debridement to ensure all dysvascular bone is removed. Punctate bleeding should be visible on all bone surfaces. An expendable bone can be completely resected and the wound treated as a soft tissue infection, substantially decreasing the duration of treatment. After debridement is completed, the dead space produced must be obliterated. A combination of local tissue closure, drains, antibiotic-impregnated spacers, and tissue transfer procedures is necessary to fill the dead space and cover exposed bone. Adequate coverage of bone is required to treat osteomyelitis. The selection of antibiotic is initially governed by the suspected pathogen. This choice is modified by sensitivity testing. A peak minimum serum bactericidal dilution of 1 : 8 is the goal of therapy. Four to 6 weeks of parenteral antibiotic therapy after the last debridement is the current standard of therapy. This type of therapy is expensive and inconvenient for patients. Outpatient services, peripheral inserted central catheter (PICC), and portable infusion pumps have helped reduce the burden of parenteral therapy. For many antibiotics, the oral and parenteral forms result in similar serum concentrations. Oral antibiotics are used for osteomyelitis in children after the initial response to parenteral antibiotics. Small clinical studies have demonstrated that oral therapy is effective in adults for susceptible organisms.36,41,70,72 Additional studies and a mechanism to ensure patient compliance are necessary before oral therapy is recommended for adult osteomyelitis therapy. Treatment of systemic conditions and hyperbaric oxygen are adjuncts to surgery and antibiotics. Attention should be paid to good nutrition, control of diabetes, and smoking cessation. Medical interventions to improve vascular status should be implemented as well. Animal studies have demonstrated hyperbaric oxygen is as effective as antibiotics in treating bone infected with S. aureus. Hyperbaric oxygen increases the intramedullary oxygen tensions in models of osteomyelitis. Increased oxygen tension is toxic to anaerobic organisms and assists phagocytic processes. Experimental models have demonstrated that antibiotics are more effective in oxygen-rich environments. Treatment regimens are 60 to 120 minutes per day with 100% oxygen at 2 to 3 atm.72,114 The use of hyperbaric oxygen is often limited by availability.
Infections of the Foot
Classification
Soft Tissue Infections
Erythrasma
Cellulitis
Necrotizing Fasciitis
Abscesses
Number
Name
Description
1
Superficial posterior
Posterior, plantar to calcaneus and plantar aponeurosis
2
Plantar
Central, between plantar aponeurosis and flexor digitorum brevis
3
Central
Central, between flexor digitorum brevis and quadratus plantae
4
Posterior dorsal
Posterior to metatarsals, between quadratus plantae and tarsal bones
5
Dorsal
Dorsal to adductor hallucis
Treatment
Debridement of Plantar Abscess
Surgical Technique
Debridement of Medial Abscess
Surgical Technique
Debridement of Dorsal Abscess
Surgical Technique
Gas Gangrene
Joint Infections

Osteomyelitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree