Imaging in Pediatric Orthopaedics
Geetika Khanna
Georges Y. EI-Khoury
Yusuf Menda
Imaging modalities have been in constant evolution since the discovery of x-rays more than a century ago. However, most of the important advances have occurred in the last 30 years. It is now hard to imagine the practice of clinical medicine or research without imaging studies. This intertwined relationship between clinical practice and imaging is very evident in orthopaedics. Radiography accounts for more than 80% of all the imaging studies performed, and it is almost always the first imaging examination that is requested before embarking on more complex studies. Other imaging modalities include fluoroscopy, magnetic resonance imaging (MRI), multidetector row computed tomography (MDCT), ultrasonography, and nuclear medicine. Radiography, fluoroscopy, and MDCT utilize x-rays generated in vacuum tubes. Nuclear medicine utilizes a form of x-rays generated by the decay of radioactive nuclei called g rays. MRI utilizes radio waves, whereas ultrasonography utilizes sound waves. Under proper and predetermined conditions, x-rays, radio waves, and sound waves can penetrate the human body and carry useful information that can be captured by appropriate detectors and be displayed either on film or on TV monitors for viewing by physicians. Within the diagnostic range, radio waves and sound waves have not been shown to produce harmful effects in humans; however, this is not the case with x-rays, especially when they are used on infants and young children (1). Because x-rays are so central to our ability to perform diagnostic work, it is essential that every physician who performs or requests imaging studies becomes familiar with the nature of this form of electromagnetic energy, understands its interaction with living tissue, and learns how to use it safely.
X-RAY TECHNIQUES
Physical Principles of X-Ray Generation.
Wilhelm Conrad Roentgen discovered x-rays in 1895 and won the Nobel Prize for Physics in 1901 for this discovery. x-Rays and visible light both belong to the electromagnetic spectrum, which has a wide range of wavelengths and frequencies. Less energetic electromagnetic waves have longer wavelengths and lower frequencies and carry radiant heat from its source. More energetic electromagnetic waves have shorter wavelengths and higher frequencies (Fig. 3-1). As the wavelength decreases, the energy of the waves increases until these waves become capable of ejecting electrons from the shells of atoms they come in contact with; this is described by the term ionizing radiation (2). By this process, electromagnetic radiation imparts energy to the tissues it interacts with. The energy dose to the tissue is defined in terms of the energy absorbed. In the past, the unit used for measuring dose was the rad (radiation absorbed dose), but now the gray (Gy) is the unit of choice; 1 Gy = 100 rad.
Electromagnetic radiation has a dual nature, exhibiting the properties of a wave as well as those of particles or bundles of energy called photons. These concepts have been postulated to explain a variety of physical characteristics of electromagnetic radiation. In contrast to sound waves, electromagnetic radiation can travel in vacuum and does not require a medium to transport it.
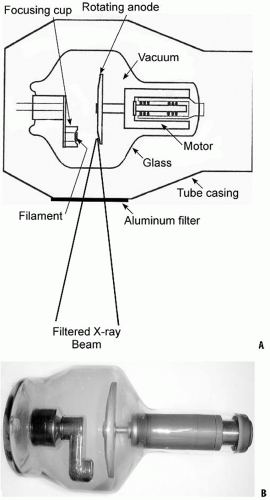
The X-Ray Tube.
In the x-ray tube, x-rays are generated when a fast and energetic stream of electrons strike a metal target, or anode. The electrons originate at the negative terminal of the tube, which is called the cathode or filament (Fig. 3-2). Electrons are decelerated by the positively charged nuclei in the target or anode, which cause the electrons to change their path and lose their kinetic energy in the form of x-rays of different wavelengths. In order to maximize the process of x-ray production, it is desirable to select a target material with a high atomic number; the nuclei of the material will have a large positive charge, capable of attracting and decelerating electrons. x-Ray tubes are typically equipped with two filaments, one large and one small, which liberate electrons when heated. The large filament is used for large exposures such as thick body parts and large or overweight patients. The area on the target
that is bombarded by electrons is referred to as the focal spot, which, in orthopaedics, should ideally be as small as possible (0.3 to 0.6 mm) in order to produce sharper images (Fig. 3-3). The energy spectrum of the emitted x-rays is determined by the voltage between the cathode and the anode.
that is bombarded by electrons is referred to as the focal spot, which, in orthopaedics, should ideally be as small as possible (0.3 to 0.6 mm) in order to produce sharper images (Fig. 3-3). The energy spectrum of the emitted x-rays is determined by the voltage between the cathode and the anode.
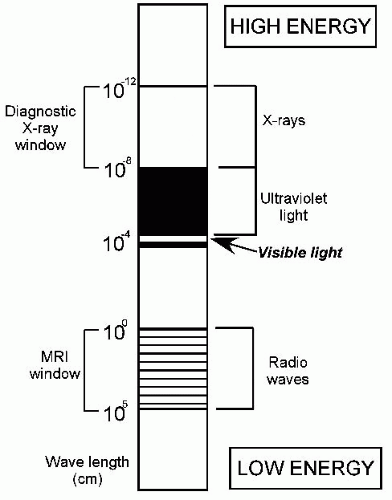 FIGURE 3-1. Diagram of the electromagnetic spectrum. Radio waves have low energy and long wavelength; x-rays have much more energy and shorter wavelength. |
The process of converting kinetic energy into x-rays is not an efficient one, and only 1% of the kinetic energy in the electron stream is converted to x-rays; the rest dissipates as heat. The ability of the x-ray tube to achieve high x-ray output is limited by the enormous amount of heat generated at the target or anode. To overcome this problem, the rotating anode was developed so that electrons do not strike the same location on the anode. This allows the x-ray tube to withstand larger amounts of heat generated during large exposures. Both the filament and the target are made of tungsten, which has a high melting point of 3370°C as well as a high atomic number (3).
In every exposure, the emitted x-ray beam consists of a wide spectrum of energies (2). The quantity of x-rays in each exposure is proportional to the number of electrons flowing from the cathode to the anode; this is measured in milliamperes. The quality, or penetrating capability, of the x-ray beam is determined by the kinetic energy of the electrons striking the target or kilovoltage setting between the cathode and anode. Electrons with high kinetic energy produce a preponderance of energetic x-rays with high penetrating power (2). Such x-rays have shorter wavelengths, higher frequencies, and more penetrating power (2).
Improving the Quality of the X-Ray Beam and Images.
With any milliampere and kilovolt setting, the x-ray beam emerges from the x-ray tube with a variety of x-rays of different wavelengths and frequencies. The interaction of x-rays with living tissue is dependent on the energy of the x-rays emitted (4). Low-energy x-rays are not diagnostically useful and are actually harmful to the patient because they are totally absorbed by the tissues and therefore fail to reach the
film or detector. To rectify this problem, x-ray tube casings are designed with filters to remove low-energy x-rays. Aluminum filters, 1 to 3 mm thick, are the most commonly used generalpurpose filters. Filtration is useful for changing the composition of the x-ray beam by increasing the ratio of x-rays that are useful for imaging to x-rays that only increase the patient’s radiation dose. This process is known as beam hardening. By filtering out low-energy radiation and by allowing high-energy x-rays to pass through, a higher proportion of the beam is capable of penetrating the patient, and carrying diagnostically useful information to the film or detector (4).
film or detector. To rectify this problem, x-ray tube casings are designed with filters to remove low-energy x-rays. Aluminum filters, 1 to 3 mm thick, are the most commonly used generalpurpose filters. Filtration is useful for changing the composition of the x-ray beam by increasing the ratio of x-rays that are useful for imaging to x-rays that only increase the patient’s radiation dose. This process is known as beam hardening. By filtering out low-energy radiation and by allowing high-energy x-rays to pass through, a higher proportion of the beam is capable of penetrating the patient, and carrying diagnostically useful information to the film or detector (4).
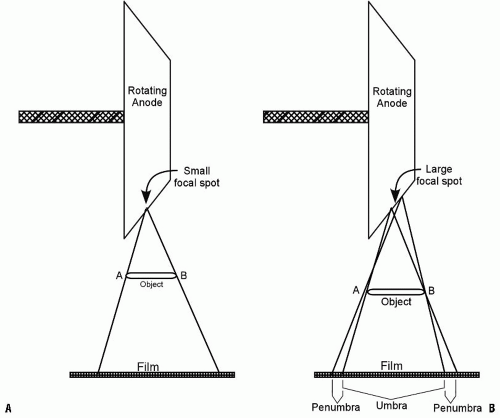 FIGURE 3-3. The effect of the focal spot size on image sharpness. A: A small focal spot produces sharp images. B: A large focal spot produces unsharp images with significant penumbra. |
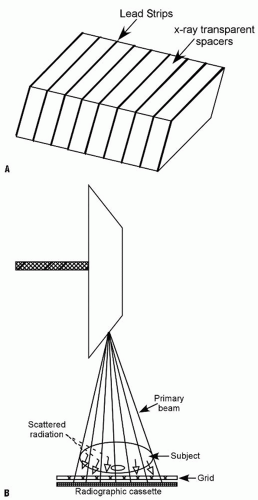
High-energy diagnostic x-rays are usually favored because the patient absorbs less radiation; however, such x-rays generate significant scattered radiation, resulting in foggy images and diminished tissue contrast on radiographs. x-Rays interacting with tissues bounce off the atoms in the tissue and are deflected from their straight path, giving rise to the scattered radiation. To control scatter and improve image quality, radiographic grids are used. The grid is the most common method for controlling scatter in medical radiography. Grids consist of thin lead strips separated by x-ray-transparent spacers (Fig. 3-4) (5). The amount of scatter is directly proportional to the thickness of the body part, and also to the field size (i.e., the area exposed). Thicker body parts produce more scatter than thinner parts. Larger field sizes also result in scatter and poorer tissue contrast on images. Thin body parts such as hands, feet, and cervical spine produce little scattered radiation and can be radiographed without a grid. Limiting the field size to the area of interest achieves two important objectives: it reduces scatter and limits the radiation to the body part of clinical interest (Fig. 3-5).
The grid is positioned between the patient and the image receptor. x-Rays traveling in a straight line carry useful information and, for the most part, pass through the transparent spacers in the grid and reach the film or detector. Scattered or deflected rays are absorbed by the lead strips and are therefore prevented from reaching the detector, where they degrade image quality (Fig. 3-4) (5).
FLUOROSCOPY
An important function of fluoroscopy is to visualize human anatomy in real time, especially during surgical or diagnostic intervention. Modern fluoroscopy systems include an image intensifier and a television monitor, but, apart from that, fluoroscopy and radiography share the same imaging components. Because physicians operate the fluoroscopic equipment, it is important that they understand the physical principles that govern the safe use
of such equipments (6). The difference between fluoroscopy and radiography is that fluoroscopy puts out a low rate of radiation exposure. However, the total radiation exposure from fluoroscopy is more than radiography. Fluoroscopic equipment is available in many different configurations for use in a wide variety of clinical applications. Mobile C-arm units are ideal for orthopaedic work. They provide a compact design, image chain angulation, and image recording devices. Mini C-arm systems are fairly inexpensive. They are designed for imaging the extremities where only low exposures are needed (6). Physicians using fluoroscopy should be cognizant of techniques that decrease radiation exposure such as pulse fluoroscopy and the “last image hold” feature that modern fluoroscopic units have.
of such equipments (6). The difference between fluoroscopy and radiography is that fluoroscopy puts out a low rate of radiation exposure. However, the total radiation exposure from fluoroscopy is more than radiography. Fluoroscopic equipment is available in many different configurations for use in a wide variety of clinical applications. Mobile C-arm units are ideal for orthopaedic work. They provide a compact design, image chain angulation, and image recording devices. Mini C-arm systems are fairly inexpensive. They are designed for imaging the extremities where only low exposures are needed (6). Physicians using fluoroscopy should be cognizant of techniques that decrease radiation exposure such as pulse fluoroscopy and the “last image hold” feature that modern fluoroscopic units have.
IMAGE FORMATION AND TRANSMISSION
Photons from the x-ray source interact with tissues and pass through the patient’s body to reach the film or detector. Variations in tissue composition give rise to differences in attenuation and spatial variations in the x-ray beam exiting the patient’s body. For air-containing organs such as the lung, x-rays pass through with only minimal attenuation, whereas most x-rays are absorbed or markedly attenuated as they pass through cortical bone. Fat attenuates x-rays more than air does, whereas water and soft tissues attenuate x-rays more than fat but less than bone. These alterations in the x-ray beam produce different responses on the film or detector. The diagnostic information on a radiograph or fluoroscopic image is obtained from the x-rays that emerge after the incident x-ray beam passes through the body (4).
IMAGING AND IMAGE TRANSMISSION IN THE DIGITAL AGE
In the last two decades, filmless imaging technologies have started to take hold, and at this time almost all imaging in the developed countries is filmless. The advances that brought about this change include faster and cheaper computers along with reasonably priced memory storage devices. In addition, the modern digital equipment manufactured by different companies can communicate information easily using a standard digital protocol called Digital Imaging and Communication in Medicine (DICOM) standard. There are several factors that may motivate radiologists and hospitals to switch to digital radiography, but the main factor is the ability to acquire high-quality images and to store and distribute them efficiently using a picture archiving and communications system (PACS). What is also important in a busy orthopaedic practice is the ability of digital radiography to accelerate patient throughput (7, 8).
Large-area flat-panel radiography detectors have been introduced during the last decade. They are now the technology of choice for acquiring high-quality images and distributing them throughout the medical enterprise rapidly and efficiently. Some studies have reported an approximate 50% reduction in the radiation dose required for skeletal imaging. There is also a large ergonomic advantage over the screen/film and computed radiography systems because there is no need for cassette handling (Fig. 3-6).
The advantages of time saving by switching to digital technology will not be fully realized unless there is improved efficiency in image management and reading. Ideally, digital images should be viewed on modern flat-panel monitors using liquid crystal display (LCD) screens with 3 million pixels (2000 × 1500).
MULTIDETECTOR ROW COMPUTED TOMOGRAPHY AND ITS APPLICATIONS
With the advent of MDCT in 1992, computed tomography (CT) witnessed a significant evolutionary advance in technology. The newer MDCT machines have rows of detectors aligned along the longitudinal axis of the patient (z axis) as well as along the transverse or axial plane (x-y axis). The advantages of this technology include unprecedented speed, increased coverage, isotropic imaging, ability to image structures that have metal hardware, and ease of interpretation. The high speed has reduced the scanning time to a few seconds.
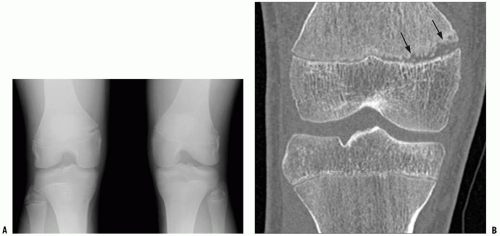
This resulted in better temporal resolution and therefore less motion blur, less need for sedation in children (Fig. 3-7), and considerable time saving in emergency situations (9).
This resulted in better temporal resolution and therefore less motion blur, less need for sedation in children (Fig. 3-7), and considerable time saving in emergency situations (9).
 FIGURE 3-6. Direct digital radiography equipment. A: The film-screen combination is substituted with a flat-panel detector. B: The image appears on a TV monitor a few seconds after the exposure. |
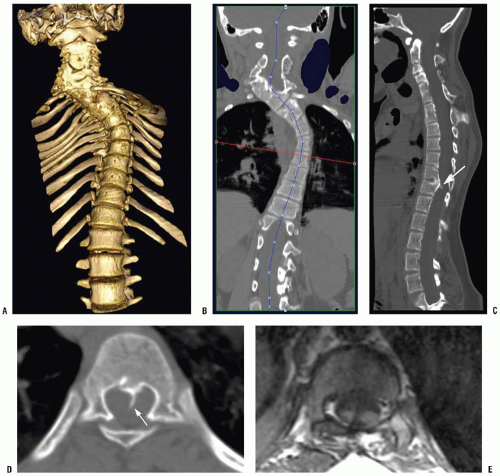
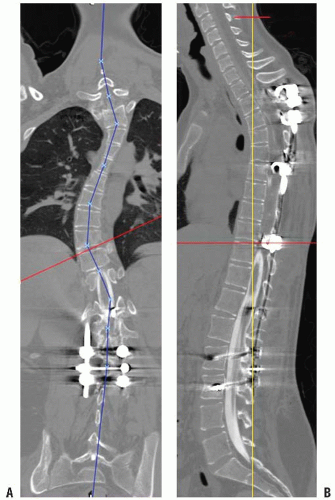
Isotropic imaging provides improved spatial resolution, the ability to obtain two-dimensional (2D) reformatted images in any arbitrary plane, and high-quality three-dimensional (3D) images (Fig. 3-8). This includes not only images in the standard sagittal and coronal planes but also curved planar reformations, which allow straightening of curved structures such as a scoliotic spine (Fig. 3-9) (10, 11). 3D surface and volume rendering are used to display anatomic relations, which are sometimes essential for surgical planning (Figs. 3-9 and 3-10).
RADIATION SAFETY IN PEDIATRIC ORTHOPAEDICS
Infants and children constitute a special subpopulation for radiation safety considerations because they are the most susceptible to radiation effects. Radiation risk is several fold higher in infants and children than in adults. It is estimated that about 7 million CT scans were obtained on children in 2007, and this number is growing by approximately 10% every year. There has been an almost 400% increase in number of cervical spine CTs obtained on children in the emergency room setting, over the period 2000 to 2006 (12). Although CT remains an extremely valuable diagnostic tool, the radiation exposure from CT has become a public health concern. The Image Gently Campaign is a recent national initiative introduced by the Alliance for Radiation Safety in Pediatric Imaging. The goal of this campaign is to change our practice by increasing our awareness to lower the radiation dose in children. The four main guiding principles issued by the Image Gently Campaign to reduce radiation exposure in children are as follows:
Reduce or “child size” the amount of radiation used. The imaging parameters such as kilovoltage and milliamperes should be adjusted to patient size when imaging children. This conforms to the ALARA principle (as low as reasonably achievable).
Scan only when necessary. If the clinical question can be answered using a modality that does not require exposure to radiation, such as ultrasound or MRI, CT should be avoided.
Scan only the area indicated. Exposure should be limited to those parts of the body that are absolutely essential to arrive at a diagnosis. When using fluoroscopy, radiation exposure can be limited by using electronic collimators to limit exposure to the area of interest only.
Scan once only. Multiphasic scans such as precontrast and postcontrast imaging should be avoided.
ULTRASONOGRAPHY
Ultrasonography is a valuable imaging technique in infants and children. The high ratio of cartilage to bone in a child’s skeleton makes ultrasonography an ideal tool for the pediatric musculoskeletal (MSK) system as compared to radiography or CT. With the increasing awareness of the deleterious effects of radiation on the growing child, ultrasonography has become an even more valuable tool. Other advantages of ultrasonography include the ability to perform real-time imaging with multiplanar capability, no need for sedation, easy portability, wide availability, and relatively low cost.
Physics.
Diagnostic sonography typically operates at frequencies between 1 and 20 MHz. The average velocity of sound in soft tissues is 1540 m/s, being higher in tissues with higher density, such as bone, and lower in tissues of lower density, such as fat. As the sound waves travel through tissues, they become attenuated because of reflection, refraction, absorption, and scattering. The reflected component gives the echo, which forms the image. The brightness of the image is directly proportional to the echo strength and produces what is called the gray-scale image. The amount of reflection that occurs at the interface between two tissues is directly proportional to the difference in their acoustic impedance. An interface that reflects most of the sound beam, such as bone, is termed highly echogenic and appears bright on the screen. In contrast, sound-transmitting structures, such as cysts, do not reflect the sound waves and appear dark or anechoic. Skeletal muscle is hypoechoic compared to adjacent fat and bone. The perimysial septae, which separate the primary fascicles within muscles, appear as parallel echogenic lines against the hypoechoic background of the muscle on longitudinal scans. Normal tendons are echogenic and exhibit a fibrillar echotexture, corresponding to the interfaces between the densely packed collagen bundles and the surrounding septa. The display of the fibrillar echotexture requires that the ultrasound beam be perpendicular to the axis of the tendon (13). If the ultrasound beam is oblique to the tendon, false hypoechogenicity is produced, which may mimic a tear. Nonossified cartilaginous epiphyses appear hypoechoic relative to the adjacent soft tissues, and usually contain fine-speckled echoes. Ossification centers within cartilage appear hyperechoic. Articular cartilage appears as a smooth, anechoic, 1- to 2-mm-thick layer.
Doppler imaging is based on the principle that when sound waves hit a moving particle, the reflected sound undergoes a frequency change (Doppler shift), which is directly proportional to the velocity of the moving object. In color Doppler imaging, the frequency change or velocity is depicted in different shades (higher frequencies being assigned lighter colors), whereas the direction, according to convention, is denoted in red for flow toward the transducer and in blue for flow away from the transducer. Power Doppler sonography is more sensitive to slow flow but lacks directional information.
Applications Developmental Abnormalities.
Developmental dysplasia of the hip (DDH) is the most common indication for MSK ultrasonography in neonates (14). In the first 6 months of life, sonography offers many advantages over radiographs as it can directly visualize the cartilaginous femoral head and surrounding soft-tissue structures such as the labrum. Though clinical hip screening detects the great majority of cases, the problem of late emergence of developmental hip dysplasia has led to the widespread use of hip sonography for the early diagnosis of this condition. A study of 7236 infants in the Netherlands showed that hip sonography had a sensitivity of 88.5%, specificity of 96.7%, a positive predictive value of 61.6%, and a negative predictive value of 99.4% (15). This study also showed that selective use of neonatal hip sonography in babies with risk factors results in a trend toward a lower rate of emergence of
late DDH cases (15). Although universal sonographic screening for DDH would detect cases that are missed clinically, the drawbacks of universal screening include cost, the potential for overtreatment, and complications such as avascular necrosis of the femoral head. In North America, neither the US nor the Canadian government believes that current evidence supports universal screening of the general population with hip sonography (16). Sonography is recommended for neonates in whom the physical examination of the hip is equivocal and for infants with risk factors for DDH. These risk factors include a positive family history, breech birth position, and conditions that can be caused by intrauterine crowding such as neonatal clubfoot and torticollis (17). The current available evidence does not recommend the routine use of hip sonography for infants with an abnormal clinical examination after 2 weeks of age (18). These infants should be referred directly to orthopaedists for treatment. Screening sonography for DDH is optimally performed when the infant is 4 to 6 weeks old. This approach reduces the rate of false-positive cases, which may be seen in the neonatal period due to physiologic immaturity of the hips.
late DDH cases (15). Although universal sonographic screening for DDH would detect cases that are missed clinically, the drawbacks of universal screening include cost, the potential for overtreatment, and complications such as avascular necrosis of the femoral head. In North America, neither the US nor the Canadian government believes that current evidence supports universal screening of the general population with hip sonography (16). Sonography is recommended for neonates in whom the physical examination of the hip is equivocal and for infants with risk factors for DDH. These risk factors include a positive family history, breech birth position, and conditions that can be caused by intrauterine crowding such as neonatal clubfoot and torticollis (17). The current available evidence does not recommend the routine use of hip sonography for infants with an abnormal clinical examination after 2 weeks of age (18). These infants should be referred directly to orthopaedists for treatment. Screening sonography for DDH is optimally performed when the infant is 4 to 6 weeks old. This approach reduces the rate of false-positive cases, which may be seen in the neonatal period due to physiologic immaturity of the hips.
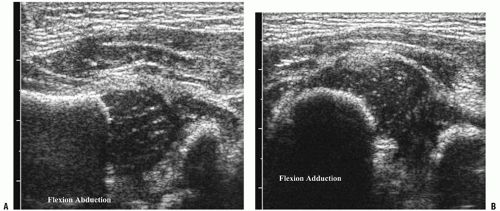
There are two major sonographic methods for evaluating the hip: the static Graf method, which emphasizes morphology, and the dynamic Harcke technique, which emphasizes stability of the femoral head (19, 20). In the static method, coronal plane imaging is performed with the infant in the supine or decubitus position, and the hip is assessed qualitatively and quantitatively (Fig. 3-11A,B). The a angle represents the bony roof, and the b angle represents the cartilaginous roof of the acetabulum. However, these angles have been shown to have poor reproducibility between examiners (21). Dynamic assessment of the hip subjects the hip to stress maneuvers to determine its stability. With the infant relaxed, the examiner can assess for ligamentous laxity and dislocatability by performing adduction with gentle stress or, for positioning and relocatability, by performing abduction (Fig. 3-12A,B). Based on the sonographic appearance of the hip, the four main classifications used are normal, immature, mild dysplasia, and more severe dysplasia with femoral head displacement (18). Once a child with hip dysplasia has been placed in a harness, sonography can be used to assess the relation of the femoral head to the acetabulum. Color Doppler imaging can determine the adequacy of blood supply to the femoral head and can identify patients at risk for avascular necrosis (22).
Ultrasonography can also be used to evaluate the nonossified structures in congenital limb abnormalities such as proximal focal femoral deficiency (PFFD), congenital dislocated or hypoplastic patella, and clubfoot (23, 24). PFFD is characterized by a variable degree of deficiency in the proximal femur. Because of its ability to identify the cartilaginous femoral head and the unossified portion of the femur, ultrasonography can be used for determining the severity of PFFD. It is particularly useful for differentiating between type A and type B PFFD, by demonstrating the presence or absence of a cartilaginous connection between the femoral head and femoral shaft. The largely cartilaginous bones of babies’ feet allow ultrasound imaging to depict them and also to determine and describe their malalignment in clubfoot and congenital vertical talus. Despite the ability to provide diagnostic information, sonography of infant feet has not become mainstream in evaluation of the foot.
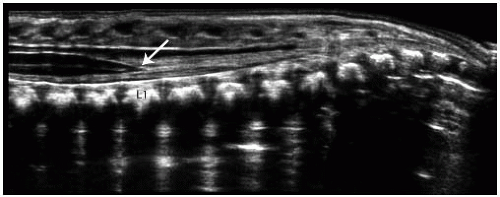
Ultrasonography is a fast and inexpensive method for ruling out the presence of a tethered cord in a child born with a sacral dimple or hairy patch, thereby avoiding an MRI that requires sedation. In the first 6 months of life, the cartilaginous elements of the posterior spinous processes permit sound waves to reach the spinal canal, thereby enabling visualization of the cord. In the newborn, the conus ends at the L2-3 level. Ultrasound can be used to evaluate the appearance and position of the conus and surrounding nerve roots in the thecal sac (Fig. 3-13). Sonographic features of a tethered cord include low-lying conus, dorsally displaced nerve roots (which may be adherent to a posterior wall of the thecal sac), echogenic fat tissue in the thecal sac distally, and lack of normal motion of the cauda equine. A normal ultrasound precludes the need for further evaluation with MRI.
Infection and Inflammation.
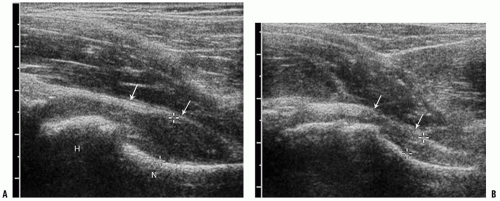
A major application of ultrasonography in pediatric MSK imaging is the evaluation of the painful hip. Ultrasonography is a safe, noninvasive, and sensitive technique for the detection of a joint effusion. Anterior parasagittal images with the foot pointed anteriorly show the normal echogenic capsule and synovium closely applied to and following the concavity of the femoral neck. Fluid that fills the hip joint causes a convex outward shape of the joint capsule, providing a confident diagnosis of joint effusion. Allowing the infant to assume a more comfortable frog-leg posture should be avoided because it allows fluid to pool posteriorly and can lead to a false-negative study. (Fig. 3-14A,B). The asymptomatic, contralateral side offers an excellent comparison. As little as 1 mL of fluid can be detected (25, 26); however, the appearance of fluid on ultrasound is nonspecific and cannot be used to differentiate a septic hip from toxic synovitis (27). Although the detection of increased blood flow on Doppler evaluation suggests septic arthritis, a normal Doppler result does not exclude the possibility of infection. Once the presence of fluid is detected, sonography can be used to guide percutaneous aspiration, in order to distinguish between toxic synovitis and a septic joint.
Ultrasonography is also the preferred diagnostic test for the evaluation of a possible foreign body, especially when the foreign body is small and radiolucent, like a wood splinter (Fig. 3-15). Wood produces posterior acoustic shadowing, whereas metal and glass demonstrate posterior reverberation artifacts. Sonography has been shown to have a sensitivity above 95% in the detection of foreign bodies (28).
In inflammatory arthropathies, such as juvenile idiopathic arthritis (JIA), ultrasonography can be used for monitoring
the inflammatory process by quantifying synovial thickening, suprapatellar effusion, and cartilage thickness (29).
the inflammatory process by quantifying synovial thickening, suprapatellar effusion, and cartilage thickness (29).
Soft-Tissue Masses.
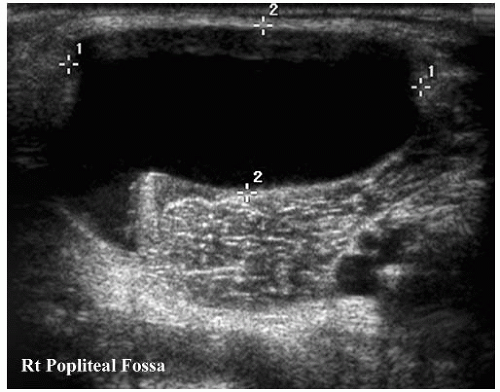
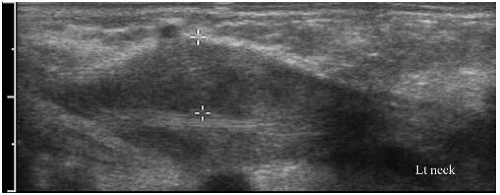
Although MRI is more accurate in characterizing and delineating the extent of soft-tissue masses, ultrasonography is a simple, noninvasive modality that can be used to evaluate suspected soft-tissue lesions and to differentiate between cystic and solid masses (Fig. 3-16). Sonographic evaluation of a soft-tissue mass is best performed with a high-resolution linear-array transducer. When evaluating a mass, the use of an acoustic standoff pad helps to improve visualization of superficial lesions. Ultrasonography can diagnose soft-tissue masses that have typical imaging features, such as cysts, fibromatosis coli, lymphangiomas, lymphadenopathy with abscess formation, and vascular malformations (Fig. 3-17) (30). When soft-tissue tumors have a nonspecific sonographic appearance, ultrasonography can be used to localize the mass and to provide guidance for needle biopsy.
MAGNETIC RESONANCE IMAGING IN THE EVALUATION OF PEDIATRIC MUSCULOSKELETAL DISEASE
Due to its excellent soft-tissue contrast MRI has become the modality of choice in the evaluation of the bone marrow, cartilage, joints, and soft tissues. The absence of ionizing radiation makes MRI particularly advantageous in the imaging of children. Its primary limitation is the relatively long scan time and the need for sedation in most children under 5 years of age.
MRI Physics.
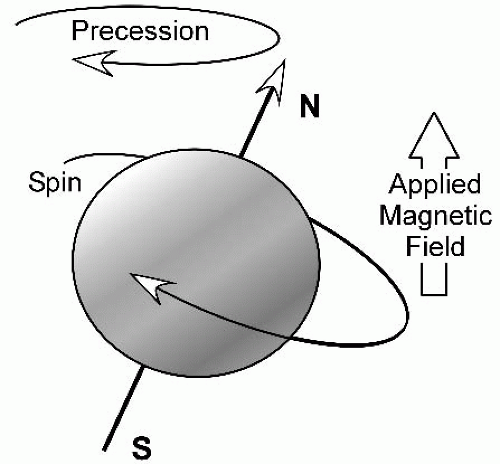
Several excellent texts addressing the physics of MRI are available. We briefly discuss the concept of T1 and T2 weighting in the following paragraphs. The hydrogen nucleus is the most commonly imaged nucleus in clinical MRI because of its abundance in biologic tissues and its favorable magnetic properties. The spinning protons act as small magnets, and when placed in a large external magnetic field, they align themselves parallel with the magnetic field (Fig. 3-18). In conventional spin-echo imaging, a radio frequency (RF) pulse is applied, which causes the protons in the tissue to flip by 90 degrees. When the RF pulse is removed, the protons realign themselves parallel with the external magnetic field. The rate at which the protons realign with the external magnetic field is called T1 relaxation (Fig. 3-19A,B). This is a tissue-specific time constant. The time required for 63% of the deflected nuclei to realign with the external magnetic field after the termination of the 90-degree RF pulse is the T1 relaxation time of that tissue. The T1 relaxation time of fat is shorter than that of water; so, on T1-weighted (T1-w) images, fat has high signal intensity, whereas water has relatively low signal intensity. T1-w images are excellent for depicting anatomic detail (Table 3-1).
T2 decay refers to loss of magnetization in the transverse plane. Immediately following the 90-degree RF pulse, all the deflected nuclei lie in the transverse plane and spin in phase; however, soon thereafter, because of interactions with neighboring nuclei, they slip out of phase. As a result, transverse magnetization decreases, and this is called T2 relaxation or decay. T2 decay is faster for fat than for water, and therefore water has a higher signal than fat on T2-weighted (T2-w) images (Fig. 3-20).
Commonly Used Pulse Sequences.
The primary imaging sequences used in MSK MRI are T1-w, T2-w, proton density, fat-saturated T2-w, or STIR (short tau inversion recovery) images and postcontrast T1-w images with fat saturation.
T1-w images are excellent for anatomic detail. They also have the highest specificity in detecting marrow pathology and posttraumatic abnormalities such as nondisplaced fractures (Fig. 3-21). Most abnormalities have a low signal intensity on T1-w sequences. A few exceptions include lesions that contain blood products, fat, high protein content, and calcifications (Fig. 3-21). Fat saturated T1-w sequences after intravenous administration of gadolinium (Gd) are used to differentiate tumor from cyst and to assess the presence of fluid collections (abscesses) in cases where osteomyelitis or soft-tissue infections are suspected.
T2-w images are most sensitive for the detection of pathology. Most lesions have high signal intensity on T2-w images, and therefore, T2-w images have higher sensitivity in the detection of pathology. Lesions that have a low T2 signal are relatively few and include mineralized tissue, fibrosis, blood products (e.g., hemosiderin), and high concentrations of Gd. Due to the normal high signal of fat on T2-w images, T2-w sequences should be performed with fat saturation. This is especially true when imaging bone marrow, where edema is much more conspicuous on fat-suppressed T2 fast spin-echo (FSE) images. Fat suppression can be achieved by applying a frequency-selective presaturation pulse, or by using an inversion recovery sequence (STIR). Inversion recovery sequences, however, have less signal-to-noise ratio than the fat-suppressed T2-w images (31). While STIR images are generally used to cover a large area in the coronal or sagittal planes, fatsaturated T2-w images are excellent for numerous thin slices in the axial plane. In the presence of metallic hardware, STIR sequences can be very helpful in reducing the metal artifacts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree