Fig. 26.1
Callistemon spp. (Australian bottle-brush plant)
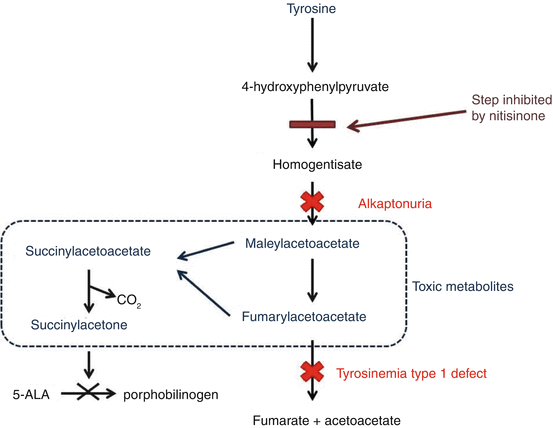
Animal toxicity studies were carried out at ICI and subsequently Zeneca, which was established in 1993 by demerging of various business units of ICI. These studies demonstrated that rats treated with NTBC developed corneal lesions due to hypertyrosinaemia and high levels of tyrosine in the aqueous humour of the eyes, which disappeared on discontinuing of the herbicide. Although the herbicide programme was abandoned (Lock et al. 1998), it was established that the hypertyrosinaemia was due to inhibition of 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme in the tyrosine catabolism pathway HPPD that converts 4-hydroxyphenylpyruvate to homogentisic acid which is implicated in the pathogenesis of ochronosis seen in alkaptonuria. NTBC was shown to be a potent, time-dependent, tight binding yet reversible inhibitor of HPPD in rats.
ICI/Zeneca’s central toxicology laboratory collaborated with Professor Lindstedt to demonstrate that NTBC, in addition to being a rat HPPD inhibitor, was a potent inhibitor of human HPPD as well. Once this was demonstrated, Professor Lindstedt requested ICI/Zeneca to supply NTBC to treat children with tyrosinaemia type I. Following the essential ethical approval, from the University of Gothenburg in Sweden and the Medical Protection Agency (Swedish regulators), ICI/Zeneca cleared the necessary legal/patent formalities, and NTBC was made available to Professor Lindstedt for clinical use. The first patient, a 2-month-old child with acute presentation of tyrosinaemia type 1, was treated in February 1991 (starting dose: 0.1 mg/kg/day, increased to 0.4 mg/kg/day) with good results.
Subsequently four more patients with sub-acute presentation of tyrosinaemia were treated (starting dose: 0.2 mg/kg/day increased to 0.6 mg/kg/day). The results, which were published in Lancet, concluded – ‘No side effects were encountered. Inhibition of 4-hydroxyphenylpyruvate dioxygenase may prevent the development of liver cirrhosis and abolish or diminish the risk of liver cancer. Normalisation of porphyrin synthesis will eliminate the risk for porphyric crises. This type of treatment may thus offer an alternative to liver transplantation in hereditary tyrosinemia’ (Lindstedt et al. 1992). The study was later further expanded, and a worldwide study was started (‘The NTBC Study’). This was coordinated by the team from Sahlgrenska University Hospital (SU), Gothenburg, Sweden, to document the effects of nitisinone treatment in tyrosinaemia type 1 patients. This was an uncontrolled, compassionate use, multicenter trial involving 96 local investigators at 87 different hospitals in 25 countries. On request, nitisinone was distributed from SU to hospitals all over the world on a compassionate use basis. The physicians/investigators sent blood and urine samples for analysis to SU at regular intervals, according to a protocol designed by the research team at SU, together with results of local laboratory tests and clinical information. The NTBC Study period was from February 1991 to August 1997. In 1994 Zeneca sublicensed NTBC to Swedish Orphan AB to commercialise NTBC. From late 1994, the distribution of nitisinone was gradually shifted from SU to Swedish Orphan AB, Stockholm, Sweden. The study patients continued the treatment after the study on a compassionate use basis. The clinical documentation submitted to the European Medicines Agency (EMA) in 2004 consisted mainly of the analysis of the results of the NTBC Study. The safety analysis also included those patients given nitisinone on a compassionate use basis, but not participating in the NTBC Study. Evidence of clinical efficacy was mainly based on the compassionate use in 207 patients enrolled in the NTBC Study. Nitisinone was administered orally twice daily, initially at a daily dose of 0.6 mg/kg bodyweight until it was clear that this dose was generally too low. From 1994, 1 mg/kg body-weight was recommended as the total daily initiation dose. The individual response to treatment was evaluated, and the dose was adjusted if considered necessary. No daily dose exceeded 3.0 mg/kg. Nitisinone treatment was always combined with a diet restricted in tyrosine and phenylalanine.
Besides the main analysis regarding the 207 patients who were included between 23 February 1991 and 21 August 1997, a complementary analysis referred to the 250 patients who were included between 1 July 1993 and 28 March 2000, after all investigators had received the recommendations of an initial daily dose of l mg/kg body-weight. Hereditary tyrosinaemia type 1-specific biochemical variables (urine and plasma succinylacetone, erythrocyte PBG synthase and urine 5-ALA), a-fetoprotein, death, liver transplants, hepatocellular carcinoma and porphyria-like crises were evaluated.
On January 18, 2002 the FDA approved (priority approval) nitisinone for the treatment of hereditary tyrosinaemia type 1. Since then, it has been marketed in the USA by Rare Disease Therapeutics (RDT) as Orfadin. On February 21, 2005, under orphan drug regulations, the EMA granted marketing authorisation under exceptional circumstances, for the treatment of hereditary tyrosinaemia type 1 (Orfadin EPAR 2009). In June 2009, EMA Committee on Human Medicinal Products (CHMP) opined that there were no remaining grounds for the marketing authorisation (MA) to remain under exceptional circumstances, and subsequently the MA was granted with unlimited validity (Fig. 26.2).