OVERVIEW
Total ankle arthroplasty (TAA) has two main design concepts: fixed/constrained and mobile bearing. Initial devices were highly constrained, whereas second- and third-generation systems are cementless, press-fit two- or three-component systems with a polyethylene insert being mobile or incorporated into the tibial or talar component (fixed).
In 2007, the US Food and Drug Administration (FDA) recognized the first mobile-bearing design with the approval of the Scandinavian total ankle replacement (STAR) prosthesis following a 7-year clinical trial. Prior to STAR approval, all TAAs recognized by the FDA were cemented designs. All other fixed-bearing, uncemented designs were not approved by the FDA. Based on FDA requirements, an ankle replacement that is produced and marketed today is called a Class II device if its design is similar to a pre-1976 implant.
1 Mobile-bearing implants have no 1976 forebearer, and thus these unconstrained devices are considered a Class III prosthesis and require randomized controlled studies for approval.
Most mobile-bearing replacements have a flat tibial component with a mobile superior surface of polyethylene meniscus creating an upper bearing surface. Mobile-bearing ankle prostheses are known as three-component replacements because there are two surfaces with motion between the three components. The geometry of the polyethylene articulates with the matched surface of the talus.
2 Medial and lateral translation of the upper bearing surface allows the polyethylene component to conform to the kinematics of the ankle, allowing maximal plantar flexion and dorsiflexion.
3Unconstrained TAA with the use of a three-component mobile-bearing prosthesis has theoretical kinematic and mechanical advantages over two-component designs, in that there is full congruency of the articulating surfaces without restriction of the rotational motion of the prosthesis.
4 Other theoretical advantages of the mobile-bearing design over fixed-bearing include decreased shear loading and decreased loading forces at the bone-prosthesis interface compared to those seen in constrained designs.
5Fixed-bearing ankle implants have one partial articulating conforming surface, whereas most mobile-bearing implants have two separate fully conforming articular surfaces.
1 Theoretically, fixed-bearing implants create more constraint through the polyethylene-talar dome interface incongruity.
This constraint also may have an effect of lowering stresses on the bone-implant interface.
6The unconstrained designs do, however, run the risk of instability, backside wear, and impingement.
7 With each iteration or phase of TAA development, the realization that deformity correction, soft-tissue balancing, and patient education are all equally important for successful outcomes.
8 To date, no clear advantage of two-component versus three-component designs has been determined in the literature.
The two major causes of early failure in mobile-bearing designs include lack of bony ingrowth and wear of the polyethylene implant. Early complication specific to all TAAs includes a potential risk for malleolar fracture secondary to bone resection of the tibia.
9,
10 and
11 Wound complications are also an issue,
12 although they decrease with increasing surgical acumen.
13Although early results have demonstrated improved functional outcomes versus ankle arthrodesis,
14 complication rates with TAA insertion are still substantial, and the procedure should be restricted to patients with valid operative criteria and surgeons with sufficient experience.
15
THE MOBILITY TOTAL ANKLE REPLACEMENT
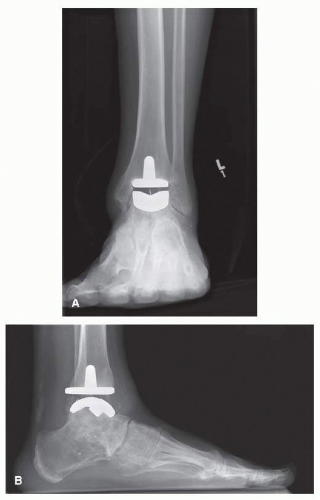
The Depuy Mobility total ankle replacement (TAR; DEPUY International) is an uncemented third-generation mobile bearing, commonly used in Europe and Canada (
Fig. 2.1A, B). The Mobility TAR is similar in appearance to the Buechel-Pappas (BP) TAA, although the Mobility has a smaller tibial stem.
16 Goldberg et al.
17 reported results of their questionnaire-based survey sent to all consultant members of the British Orthopaedic Foot & Ankle Society. The Mobility prosthesis was the most commonly used one among 62% of all surgeons in the United Kingdom. The use of this prosthesis has also been published in the literature in the Swedish Ankle Arthroplasty
13 and New Zealand Ankle Arthroplasty registries.
23The tibial component of the Mobility has a flat plate that provides both posterior and anterior cortical support.
16 The talar and tibial components are porous-coated cobalt chrome, while the meniscal bearing insert is ultrahigh-molecular-weight polyethylene (UHMWPE). The tibial component has a nonmodular intramedullary stem, while the talar component is cylindrical.
1 The talar dome is press-fit into three prepared grooves within
the talus: one central sulcus with two lateral fin grooves. The UHMWPE insert has fully congruent surfaces with both components making it semiconstrained. The polyethylene is narrower superiorly to prevent edge loading and reducing wear,
18 whereas the BP does not have the same superior narrowing.
This prosthesis is inserted through an anterior approach. Extramedullary instruments are used for bone resection and positioning the components so that the polyethylene moves minimally on the tibial plate, while the largest amount of motion occurs between the insert and the talar component. The anterior cortical bone window, created for the intramedullary peg, is replaced at the end of the procedure.
Wood and Rippstein
19 detailed the early results of 200 Mobility TARs in 2008: 100 consecutively performed TARs from the Schulthess Clinic and 100 from Wrightington Hospital, Wigan, were evaluated. The follow-up was at an average of 36 months (range, 24 to 50). In 20% of the cases, a gastroc-soleus lengthening was performed, while 8% of the cases underwent other adjunct procedures. Complications included intraoperative malleolar fractures (6%), delayed wound healing (4%), and late medial malleolar fractures (2%). One early infection occurred. Revision to an arthrodesis or a change of either component was required in five patients because of aseptic loosening. A technical error in one patient led to subluxation of the insert.
Lee et al.
20 retrospectively compared the perioperative complications of the 30 Mobility TARs inserted from May 2008 to June 2009 with those of a matched cohort of patients who had previously received a Hintegra ankle. There was no statistically significant difference in early complications. There was a tendency toward medial malleolar fracture with the insertion of the Mobility prosthesis.
Jackson et al.
21 described the early outcomes in comparing the Mobility TAR to previous STAR ankles performed. Sixtyeight Mobility TARs were compared with a similar number of STAR prostheses. There were no significant differences in operative time (63 vs 65 minutes), no differences in intraoperative complications (0 for both groups), and no deep infections were reported, with minimal difference in hospital stay (4.3 vs 5.1 days). The survival curve of both prostheses showed no revision surgery being required at up to 3 years.
21 (See
Table 2.1 for mobility studies.)
THE HINTEGRA TOTAL ANKLE PROSTHESIS
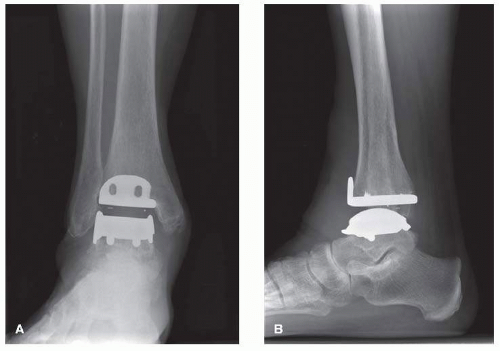
The Hintegra TAR (Integra Lifesciences) prosthesis was developed in Lyon, France, and was designed in 2000
2 (
Fig. 2.2A, B). The Hintegra is a third-generation implant, which provides inversion-eversion stability.
22 As the distal tibia metaphyseal cancellous bone grows increasingly weaker more than a few millimeters proximal to the joint, an important element in the rationale of the Hintegra prosthesis design was minimal tibial bone resection.
22The Hintegra TAR has been used since 2000 in Europe, since 2004 in Canada and Korea, and since 2005 in Brazil. The use of this prosthesis is also documented in National Arthroplasty Registers of Finland, Sweden, Norway, and New Zealand.
13,23,24,56It consists of a flat tibial component, a polyethylene inlay, and a convex conic talar component with a smaller medial radius. Both the tibial and talar components employ shields for screw fixation.
22 On the other hand, screw fixation was required to compensate for the lack of stems (BP-type implants) or anchorage bars (STAR prosthesis). In the early postoperative phase, before bone ingrowth provides adequate stability, loosening of the screws could be possible.
25 Unique about this prosthesis is the anterior tibial flange to reduce postoperative heterotopic ossification and soft-tissue adherence.
26The mobile-bearing polyethylene insert provides axial rotation and flexion-extension.
22 The tibial component has peaks on the flat surface as well as the anterior phalange with screw holes for fixation. The tibial component is flat with a 4° posterior inclination angle approximating the normal distal tibial surface.
1,22 The anterior shield of the tibial component is designed to minimize stress shielding, while the medial and lateral rims of the talar component stabilize the insert. The talar component also has screw holes, and the radius is smaller medially than laterally.
1 Much like the Mobility TAA, the superior surface of the polyethylene insert is smaller to prevent tibial impingement.
26In 2004, Hintermann et al.
22 described their experience with 122 Hintegra TARs. Of 122 TARs, 8 had to be revised: 4 due to aseptic loosening of at least one component, 1 due to dislocation of the insert, and 3 due to other complications. They reported that 84% of the TARs were satisfied. American Orthopaedic Foot and Ankle Score (AOFAS) improved from 40 points preoperatively to 85 points postoperatively. Eighty ankles (63%) were completely pain-free. Radiographically, migration of the talar component was noted in two ankles with no tibial components showing any signs of loosening (mean follow-up, 18.9 months).
Using the same series in 2010,
27 they expanded their cohort to 340 primary TARs. The overall survivorship was 97.9% for the talar component and 98.8% for the tibial component in this increased sample after 6 years. Four ankles were revised (one for loosening, three for pain) and three ankles were revised to an ankle arthrodesis. The AOFAS improved from 42.1 preoperatively to 78.6 at follow-up. Of the total, 205 ankles (60.5%) were completely pain-free.
In a level IV study in 2009 by Hintermann et al.,
28 30 ankles in 28 patients with a minimum follow-up of 36 months (average, 55.6 months), who were converted from an arthrodesis to a TAR, were reviewed. In 29 TARs in 27 patients, the AOFAS improved from 34.1 preoperatively to 70.6 at their latest followup. Five ankles were completely pain-free, 21 were moderately painful, and 3 remained painful. Radiographically, the tibial component was not stable in one and the talar component had migrated in four ankles (two of which were symptomatic).
In
Foot & Ankle International (2009), Lee et al. retrospectively evaluated their early perioperative complications from their first 50 Hintegra TARs. The first 25 patients formed group “A” and the last 25 formed group “B.” Zero major wound complications and three minor wound complications were seen in each group. One deep infection was found in group A, which required implant removal and two-stage revision. Four patients sustained intraoperative malleolar fractures in group A and only one in group B. Coronal malposition of the tibial component occurred in three cases in group A and two in group B. Increased sagittal slope of the tibial component occurred in two cases in group A and three in group B. Four patients in group A and three in group B had anterior translation of the talar component compared to the tibial component.
25Kim et al.
29 presented their early results of 55 Hintegra implants with more than 12 months of follow-up (range, 13 to
49 months). Postoperative complications included one intraoperative fracture of the medial malleolus, one medial malleolar stress fracture 6 weeks after surgery, and one case of deep fungal infection that was converted to arthrodesis. Four TARs were revised because of loosening of the tibial component and three more due to polyethylene dislocation. The Kaplan-Meier cumulative survival rate was 90.9% at 12 months and 87.8% at 49 months postoperatively.
29Hintermann et al.
30 noted that in the last 20 years TAR has become a viable alternative to arthrodesis for end-stage osteoarthritis of the ankle. To date, however, there are no reported results of revision arthroplasty for salvage of a failed ankle replacement. Based on the authors’ experience, prosthetic components with a flat undersurface are most likely to be able to find solid support on remaining bone stock. The first 83 cases (79 patients [46 men, 33 women], average age of 58.9 years, range of 30.6 to 80.7 years) with an average follow-up of 5.4 years (range of 2 to 11 years) showed good-to-excellent results in 69 cases (83%), a satisfactory result in 12 cases (15%), and a fair result in 2 cases (2%), and 47 patients (56%) were painfree. Primary loosening was noted in three cases; of these, two cases were successfully revised by another TAR and in one case with arthrodesis. Another case with hematogenous infection was also revised by arthrodesis. At the last follow-up control, two components were considered to be loose and the overall loosening rate was thus 6%. The authors concluded that this series has proven that revision arthroplasty can be a promising option for patients with failed total ankle prosthesis. The most challenging issue is the solid anchoring of available components on residual bone. (See
Table 2.2 for Hintegra studies.)
THE STAR
The first design was introduced by Kofoed
31 in 1978, with the first prosthesis implanted in 1981, which consisted of a metallic talar component that covered the medial and lateral talar surfaces, articulating with a polyethylene tibial component (two-component congruent unconstrained design) (
Fig. 2.3).
31 Both were initially fixed with bone cement.
32 The STAR is one of the most widely used implants in North America and lies in sharp contrast to semiconstrained system like the Agility, in that there is less bone resection while the syndesmotic structures are maintained.
33 The tibial and talar components are made of a cobalt-chromium alloy, and the polyethylene is an unconstrained mobile-bearing design. The polyethylene itself is a UHMWPE.
31The STAR is the only FDA-approved third-generation implant in the United States.
1 The STAR implant design calls for resurfacing of the medial and lateral talar facets without syndesmotic arthrodesis. The STAR instrumentation is extramedullary and is designed to resect less bone from the tibial and talar surfaces when compared to the Agility.
34,35The current generation of STAR in use in North America is an uncemented cobalt-chromium, unconstrained three-component mobile-bearing design with a titanium plasma-sprayed ingrowth surface without hydroxyapatite (HA).
36The three components that form the STAR prosthesis are as follows: (1) a tibial component with a highly polished flat articulation surface and two cylindrical fixation bars on the proximal side of tibia to anchor the implant in the subchondral bone of the tibia; (2) a talar component, available in different sizes for right and left, with a ridge running anteroposteriorly in
the middle of the gliding surface to guide the mobile bearing; and (3) a UHMWPE mobile-bearing sliding core, the flat surface of which articulates with the tibial component, while the concave-shaped underside articulates with the convex-shaped talar component.
37 The anteroposterior articulation is guided by the longitudinal ridge on the talar component and the matching longitudinal groove in the underside of the mobilebearing sliding core. Dorsiflexion and plantar flexion at the meniscal-talar interface, but no talar tilt, are allowed. Rotation is allowed at the (flat) meniscal-tibial interface.
37 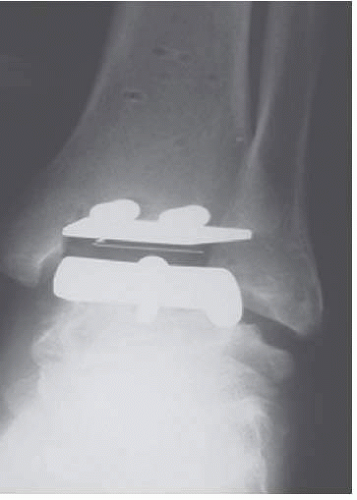


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access