Future Developments
The senior author (SSB) has been fortunate to participate in the development of shoulder arthroscopy from the very beginning. This experience has produced a unique perspective on where we have been and where we might be going. Naturally, we never really know where the future will take us until we get there, but certain clues can lead us into close proximity.
WHERE WE’VE BEEN
We believe that the development of arthroscopic shoulder surgery was rapidly accelerated when we began to think of the shoulder in mechanical terms. From the standpoint of the rotator cuff, the ability to model the shoulder in terms of balanced force couples and transmission of a distributed load across a cable-like band (suspension bridge analogy) helped us to understand how to repair the mechanical derangements that were brought about by disruption of the rotator cuff insertions. Basic biomechanical research and bench testing allowed us to develop implants and instruments to arthroscopically create mechanical constructs that were significantly stronger than the disruptive forces to which these constructs were subjected.
In the realm of instability, we recognized the critical importance of glenoid and humeral bone deficiency. Significant bone deficiencies have biomechanical consequences, and the adverse consequences of bone loss can be so severe that they cannot be overcome by simple repair. Such biomechanical deficiencies often demand a bone graft to provide a large enough articular arc to restore stability. The development of criteria to recognize and appropriately address bone deficiency in instability has allowed for a biomechanically based algorithm that covers the spectrum from arthroscopic capsulolabral repair to open Latarjet reconstruction with coracoid bone graft (Table 25.1).
WHAT WORKS AND WHAT DOESN’T
Suture anchors were introduced to the orthopaedic market in 1991. The senior author (SSB) was initially skeptical of suture anchor fixation but became convinced of its superiority over transosseous cuff repair after conducting biomechanical studies of these two types of fixation (1,2). These studies demonstrated that suture anchors provided superior fixation in metaphyseal bone, whereas transosseous sutures would routinely cut through that bone in laboratory testing. In fact, it was shown that transosseous bone tunnels needed to be placed in the cortical bone, distal to the metaphyseal bone, to have sufficient strength to avoid bone cut-through of the sutures with cyclic loading.
In the early 1990s, the senior author (SSB) developed a guide system for performing arthroscopic transosseous rotator cuff repair (Fig. 25.1) and actually performed approximately a dozen of these procedures. In order to get good fixation in cortical bone, a new portal was developed that approached the humerus distal to the axillary nerve (3). Although fixation was good at that level and the patients who underwent this procedure generally did well, we abandoned it in favor of suture anchors because the approach below the axillary nerve seemed potentially too dangerous and the fixation was no better than with suture anchors.
TABLE 25.1 ALGORITHM FOR MANAGING ANTERIOR INSTABILITY
| 1. Intraoperative arthroscopic assessment |
| Determine glenoid bone loss and Hill-Sachs depth with a calibrated probe |
| Assess for an engaging Hill-Sachs lesion |
| Evaluate for a SLAP lesion and repair if present |
| 2. Definitive surgery |
| Glenoid bone loss <25% |
| Arthroscopic repair: |
| Bankart repair, and Remplissage for Hill-Sachs ³4 mm in depth |
| Glenoid bone loss ³25% |
| Open Latarjet after arthroscopic SLAP repair if present |
| Deep (>4 mm) Engaging Hill-Sachs without substantial glenoid bone loss |
| Arthroscopic Bankart and remplissage for patients Open Latarjet for patients with high physical demands vs. Arthroscopic Bankart and remplissage for patients with low physical demands |
| Engaging Hill-Sachs with glenoid bone loss ³25% |
| Open Latarjet |
We never even considered changing the transosseous tunnel’s exit point to a more proximal and accessible location in metaphyseal bone, above the level of the axillary nerve, because the suture anchor’s fixation strength was far superior to transosseous fixation in metaphyseal bone. Once we had identified the strongest fixation construct (suture anchor fixation), there was no logical reason to use the weaker construct (metaphyseal transosseous fixation). We simply switched over to the stronger suture anchor techniques.
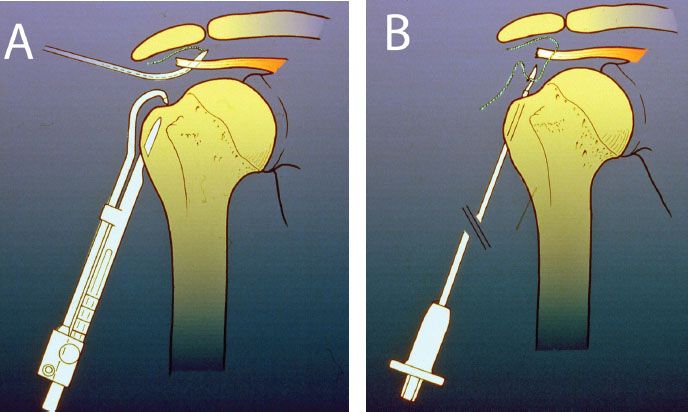
Figure 25.1 Schematic of a transosseous guide system for rotator cuff tear developed by one of the authors (SSB) in the early 1990s. A: A suture is passed through the rotator cuff and a bone tunnel is created using a subaxillary nerve lateral portal. B: The previously placed rotator cuff suture is retrieved through the bone tunnel with a snare device.
Today, some surgeons are advocating a return to “the gold standard” of metaphyseal transosseous bone tunnel fixation. There are a couple of things wrong with the logic of that position. First of all, it would be a giant step backward, reverting to a procedure that was the “gold standard” of Dr. Charles Neer more than 50 years ago. Secondly, it ignores the biomechanical research that has shown the inferiority of that technique. Finally, from a personal perspective, we’ve “been there and done that,” and we abandoned that technique for a stronger and more reliable suture anchor technique.
BIOMECHANICAL WORKS IN PROGRESS
There is an intriguing biomechanical enhancement technology that has begun in arthroscopic shoulder surgery, and that involves the augmentation of current fixation techniques with self-reinforcing systems.
Self-regulating systems have been around for a long time in the automotive industry, where there are self-balancing engine components and self-centering mechanical systems. However, biomechanical self-regulating mechanisms have only recently been identified and utilized. Specifically, the suture-bridge technique of rotator cuff fixation by means of linked bridging sutures between two rows of suture anchors exhibits self-reinforcement characteristics (4).
The term self-reinforcing refers to the fact that, the harder one tries to make the system fail, the stronger it becomes. One example of a self-reinforcing system that most surgeons are familiar with is the Chinese finger trap (Fig. 25.2). In the case of the Chinese finger trap, the harder one pulls in an attempt to disengage it from the finger, the tighter it grips the finger. In effect, it harnesses a potentially destructive force to make itself stronger. This is a very useful characteristic, particularly for a biologic repair construct such as a rotator cuff repair.
We first noted the self-reinforcing attributes of bridging double-row rotator cuff repair about 4 years ago while testing this fixation technique on cadavers in the biomechanics lab (5). We noted that the yield load approached the ultimate load, in contradistinction to nonlinked double-row repairs. The reason this happens is that the suture-bridge fixation functions much like a Chinese finger trap.
In the unloaded situation, the suture bridge forms a rectangle of fixation around a segment of rotator cuff tendon (Fig. 25.3A). Under load, this rectangle changes shape into a parallelogram with a decreased height of the construct and an increased normal force perpendicular to the tendon. The increased normal force translates into increased friction at the tendon–bone interface because of the relationship f = mN. As the normal force increases, the frictional force increases. Also, because the height of the parallelogram continues to decrease under load, the tendon becomes wedged progressively more tightly under the sutures (Fig. 25.3B). We think that the improved clinical results that are being reported with suture-bridge techniques are at least partly due to the enhanced fixation afforded by this self-reinforcing mechanism.

Figure 25.2 A linked double-row rotator cuff repair can be likened to a Chinese figure trap in which a distractive force (tensile load to the rotator cuff) results in further compression of the fingers (rotator cuff footprint).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







