Electrical Activation of the Neuromuscular System
Action potentials, and therefore muscle contractions, can be generated with electrical current. Electrical current, applied to excitable tissue through a pair of electrodes, creates a localized electric field that depolarizes the axonal membranes of nearby neurons. If the depolarization reaches a critical threshold, an influx of sodium ions from the extracellular space to the intracellular space produces an action potential that propagates from the site of stimulation and has the same effect as a naturally generated action potential. When the action potential reaches the terminals of the axon, neurotransmitter is released and, in the case of motor neurons, muscle fibers contract.
NMES systems generally operate by activating motor neurons rather than the muscle directly, even though NMES is commonly referred to as “muscle stimulation.” It is easier to stimulate a nerve than a muscle. The stimulus threshold for a neural tissue, the lowest level of charge that will generate an action potential, is much less than the threshold for muscle fibers (
1). While it is possible to generate action potentials in muscle fibers directly, it is not usually done because of power consumption considerations.
This means that for NMES to be applicable in a given patient, their lower motor neurons must be intact from the anterior horns of the spinal cord to the neuromuscular junctions of the target muscles.Damage to lower motor neurons prevents the successful application of NMES in patients with polio, late-stage amyotrophic lateral sclerosis (ALS), and peripheral nerve injuries (e.g., brachial plexus injury). In addition, patients with disorders at the neuromuscular junction or muscle tissue (e.g., muscular dystrophies) are ineligible for NMES applications. NMES may be used when the lower motor neurons are excitable and the neuromuscular junction and muscle are healthy, as is usually the case in patients with spinal cord injury (SCI), stroke, traumatic brain injury, cerebral palsy, or multiple sclerosis. To date, most motor neuroprostheses have been targeted toward the SCI population.
Large axons are easier to stimulate than small axons. Large-diameter axons, which innervate the larger motor units, have lower stimulus thresholds than small-diameter axons (
2). This is because the wider spacing between the nodes of Ranvier in large axons produces larger induced transmembrane voltage changes. The activation of large motor units before small motor units is known as reverse recruitment order and is the opposite of the physiological size principle, where small motor units are initially recruited, followed by larger motor units. In spite of this reverse recruitment order, it is still possible to produce graded muscle contractions with NMES.
Because large-diameter axons have lower stimulus thresholds, type II muscle fibers (fast-fatiguing) are preferentially recruited over type I muscle fibers (fatigue-resistant). Fatigue resistance is highly desirable for most functional NMES applications. Unfortunately, disuse atrophy tends to convert type I to type II fibers (
3). However, long-term use of NMES can reverse muscle atrophy and convert type II fast-fatiguing fibers to type I fatigue-resistant fibers (
4,
5). Therefore, all current neuroprosthetic NMES applications use some form of muscle conditioning regimen to build and maintain fatigue-resistant muscle.
Axons near the stimulus source are easier to stimulate than axons farther away. The electric field diminishes with distance from the stimulus source; as the distance between the electrode and the axonal fiber increases, the stimulus threshold also increases (
1). Less current is required to stimulate neurons in the proximity of the stimulating electrode because the transmembrane potentials generated by the electrical current are largest in the axons close to the stimulating electrode. Therefore, to improve the selectivity of stimulation, electrodes are placed as close as possible to target muscles or nerves.
The strength of a muscle contraction produced by NMES can be modulated by manipulating three stimulus parameters that characterize the wave of current pulses: pulse frequency, amplitude, and duration. If the pulse frequency is too low, the muscle responds with a series of twitches. As the pulse frequency is increased, the twitches begin to overlap and build on one another, producing a smooth or “fused” contraction. This cumulative effect of repeated stimulus pulses within a brief period of time is known as
temporal summation. Higher stimulus frequencies produce stronger muscle contractions up to a maximum, but also make a muscle fatigue more rapidly than lower-frequency stimulation. Therefore, the ideal stimulus frequency is the lowest frequency that will produce a fused contraction, and depends on the muscle fiber type and the manner in which the stimulation is being delivered (e.g., surface vs. implanted electrode). In upper extremity applications that use implanted electrodes, a stimulus frequency of 12 to 16 Hz has been found to be the minimum required for producing fused contractions if the muscles have been conditioned to have relatively long-duration twitches. The strength of a muscle contraction may also be increased by increasing the pulse amplitude and/or pulse duration, which effectively increases the electric charge per pulse (
6). The greater the electric charge, the larger the electric field and broader the region of activation. In this way, more axons and more motor units are activated, an effect known as
spatial summation. In most neuroprostheses, the strength of muscle contraction is controlled by modulating the pulse amplitude or pulse duration while keeping the pulse frequency constant and as low as possible to avoid premature fatigue of muscles.
Safe Stimulation of Living Tissue
Safe stimulation waveforms and electrode materials have been experimentally established. For NMES applications using implanted electrodes, a
current-regulated stimulator with a
balanced biphasic stimulus waveform should be used. With implanted electrodes it is important to use stimulus parameters (pulse amplitudes and durations) that are appropriate for the dimensions and material composition of the electrode so that the charge density per phase remains within the established safe limits, thereby preventing electrode corrosion or dissolution of metal ions (
1). With current-regulated stimulation, the current is directly controlled (current is the charge delivered per unit time). Therefore, the quantity of charge delivered per stimulus pulse can be maintained within safe limits. Improper stimulation can also cause tissue damage by producing irreversible electrochemical reactions at the electrode-tissue interface. Irreversible reactions can be avoided with charge-balanced biphasic stimulus waveforms. Biphasic waveforms consist of a repeating current pulse that has a cathodic (negative) phase followed by an anodic (positive) phase. The first, or primary, phase elicits an action potential in nearby axons, and the secondary positive pulse balances the charge of the primary pulse. The purpose of the secondary pulse is to reverse the potentially damaging electrochemical processes that occur at the electrode-tissue interface during the primary pulse, allowing neural stimulation without causing tissue damage (
1).
For NMES applications using surface electrodes, other safety factors need to be considered. For example, the electrode-tissue contact area is often not a constant as surface electrodes often pull away from the skin or dry out. If a current-regulated stimulator is used in this circumstance, the reduction in contact area will cause high current densities and can result in burning the skin and underlying tissue. However, with a
voltage-regulated stimulator, the magnitude of current delivered to the tissue is dependent on the impedance at the electrode interface (current = voltage/impedance). If the impedance at the electrode-skin interface increases because the electrode dries or loses strong contact with the skin, then the current delivered will decrease, thereby reducing the possibility of skin burns due to high current densities. Although this may be safer, a major disadvantage of voltage-regulated stimulation is that the muscle contraction produced is more variable because the changes in impedance lead to changes in applied current. Regardless of whether current-or voltage-regulated stimulators are used, even with good electrode-skin contact, burning of the tissue can occur if the current densities are too high. Therefore, surface stimulation should be used with caution and with frequent examination of the skin when applied to patients with impaired sensation or cognition.
Table 72-1 summarizes the principles of NMES and their practical ramifications when applying NMES systems.
NMES System Configurations and Electrode Types
At least two electrodes are required to produce a current flow in NMES applications. One electrode, often referred to as the active electrode or cathode, is placed near the peripheral nerve or muscle motor point to be stimulated. The other electrode, known as the return electrode or anode, may be placed in a remote area near less excitable tissue (such as tendon or fascia), or it may be positioned close to the active electrode to confine the electric field to a small region of activation and thereby achieve more selective muscle activation. Additional electrodes are needed to achieve coordinated activation of multiple muscles. Multichannel NMES systems stimulate multiple muscles simultaneously and use either a bipolar or monopolar arrangement of electrodes. Bipolar multichannel systems require a return electrode for each active electrode; so for each muscle or nerve to be activated, a pair of electrodes (cathode and anode) is needed. This bipolar arrangement may allow greater selectivity of activation (
7), but requires more electrodes and leads. Monopolar electrode systems reduce the number of electrodes and leads by
using only one remote return electrode with an active electrode placed near each motor point or nerve targeted for excitation.
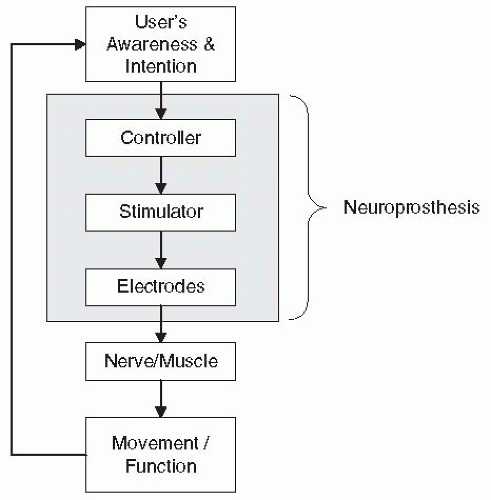
Functional NMES systems consist of at least three components: electrodes, a multichannel stimulator, and a controller (
Fig. 72-1). The electrodes deliver the electrical current pulses to the excitable tissue, the stimulator generates the current waveforms for multiple cathodes, and the controller regulates the stimulation according to the user’s intent. A controller may be as simple as a switch that the patient hits to turn the stimulation on and off, or may incorporate sensors for recording patient-elicited biopotentials (e.g., EMG or EEG) and use those as signals to regulate the stimulation. Depending on the way these three components are arranged with respect to one another, NMES systems can be categorized as surface, percutaneous, or implanted. In surface systems, the electrodes, the stimulator, and the controller are external to the body. In percutaneous systems, the electrodes are implanted near a muscle or nerve with leads that pass through the skin to an external stimulator with a separate or integrated external controller. In implanted systems, the electrodes and stimulator are implanted and the controller components are either all external or some are implanted and some are external. To date, there is no neuroprosthesis available that has all three components completely implanted, although several research programs are moving in that direction (
8,
9).
Surface NMES systems (sometimes called transcutaneous systems) use electrodes that are placed on the skin and connected with flexible cables to a stimulator, which may be worn around the waist, the arm, or the leg. Usually, a sensor or switch that controls the stimulation is also connected to the stimulator. Surface electrodes are readily available in a variety of sizes from many manufacturers. The electrodes are placed on the skin over the nerves or over the “motor points” of muscles
to be activated. The motor point is the site of stimulation that produces the strongest and most isolated contraction at the lowest level of stimulation. Surface NMES systems offer several distinct advantages: (a) the electrodes are generally easy to apply and remove, (b) the stimulation technique is noninvasive and therefore reversible, (c) the use of surface electrodes can be readily learned and applied in the clinic, and (d) stimulators and surface electrodes are relatively inexpensive and commercially available. Stimulation with surface electrodes is the most widely used technique for therapeutic applications, and has been successfully employed to produce standing, stepping, and grasping motions. However, there are several disadvantages to using surface NMES systems: (a) they cannot produce isolated contractions of small or deep muscles, (b) movement of the target muscle under the skin as the stimulated limb moves can change the proximity of the electrode to the target muscle/nerve, resulting in inconsistent muscle contraction and force production, (c) daily doffing and donning the electrodes can complicate use, especially if electrode positions vary slightly from day to day, (d) in many cases, cutaneous pain receptors are stimulated and patients with preserved or heightened sensation may find it difficult to tolerate, and (e) the system may draw unwanted attention, especially if there are multiple stimulus channels and many cables. These disadvantages have motivated the design of systems with implanted electrodes.
Percutaneous systems use electrodes that are implanted into muscles or near nerves, and have leads that pass through the skin and connect to an external stimulator. Percutaneous electrodes can activate deep muscles, can provide isolated and repeatable muscle contractions, and are less likely to produce pain during stimulation because they bypass the sensory afferents in the skin. Percutaneous electrodes are often formed from a multifilament lead within a single insulator that is wound into a helical configuration to produce maximum flexibility (
10,
11). Percutaneous intramuscular electrodes are inserted into the target muscle with a hypodermic needle. The needle is withdrawn, leaving the electrode in place and the lead exiting the skin. The exit sites on the skin are protected with a bandage and must be cleaned, dressed, properly inspected, and maintained to reduce the risk of complications (
12). A large surface electrode is often used as the return electrode. Percutaneous systems provide a minimally invasive technique for investigating the feasibility of restoring functional muscle contractions without having to prematurely subject research participants to implantable system surgery. Percutaneous systems have served as precursors to fully implanted systems (
13) and have provided function in some individuals for periods of years (
14). Various studies have reported on the longevity of percutaneous electrodes (
11,
12,
15,
16,
17). Although the failure rate due to breakage is low during the first few months post implantation, the cumulative 1-year failure rate can vary between 56% and 91% (
12,
17), but this depends on many factors including the type of percutaneous lead, lead-routing technique, and the muscles implanted. Other potential complications of percutaneous electrodes include formation of granulomas from retained electrode fragments and electrode-related infections,
which are treated with oral antibiotics or minor outpatient surgical procedures (
12).
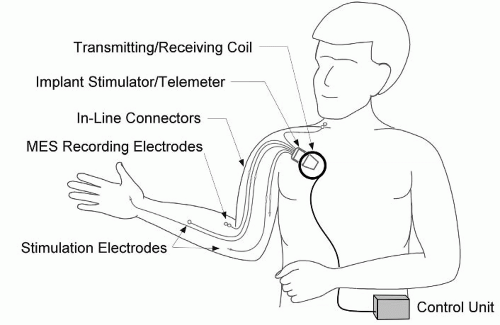
Implanted NMES systems are designed for long-term use. Both the electrodes and the stimulator are implanted, but the controller is external for most implanted systems, to date. Communication between the controller and stimulator is through radio-frequency (RF) transmission; therefore, no leads pass through the skin. Because the stimulator both receives RF commands and generates stimulus pulses, it is often called a receiver/stimulator. Implanted electrodes with insulated lead wires are connected directly to the implanted stimulator with inline connectors, which permit the surgical removal and replacement of individual electrodes, if necessary, without removing the stimulator. The electrode leads are larger than percutaneous leads because they need to be more robust and resistant to failure given the intention to make long-term use of them. In most implanted system configurations the circuitry of the stimulator is sealed in a titanium enclosure, which serves as the return electrode. Depending on the application, the stimulator is implanted in the upper chest or abdomen. The stimulator receives power and control instructions through RF telemetry from an external controller. The RF link allows the stimulator to be fully passive with no active battery, thereby eliminating the need for replacement of the implanted stimulator because of battery failure. The telemetry link requires no wires through the skin; rather, a circular coil (antenna) connected to the controller with a cable is taped to the skin over the implanted stimulator. The controller receives information from the user through switches, sensors, an implanted joint angle sensor, or biopotential electrodes to determine the user’s intention and deliver stimulation accordingly. The controller may be worn on the body or carried on a user’s wheelchair. For long-term clinical application, implanted systems provide major advantages over other systems including improved convenience, cosmesis, reliability, and repeatability.
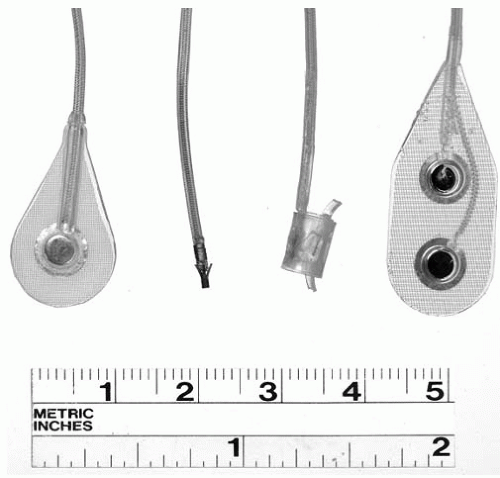
A variety of electrodes may be used with implanted NMES systems (
Fig. 72-2). Epimysial electrodes are sutured directly to the epimysium on the muscle surface (
18); intramuscular electrodes are inserted directly into a muscle belly (
19); epineural electrodes are sutured to the connective tissue surrounding a motor nerve (
20); and nerve helix or cuff electrodes are implanted around a nerve (
21,
22,
23,
24). Epimysial electrodes have proved to be durable in upper and lower extremity applications (
25,
26), and are especially useful for activating broad, superficial, or thin muscles. Intramuscular electrodes allow activation of deeper or smaller muscles, such as the intrinsic muscles of the hand. Nerve-based electrodes are used when it is difficult to access the target muscle directly or when more complete muscle recruitment can be obtained by stimulating the nerve (
27).
A unique configuration of implanted NMES system is the injectable microstimulator (BION), which is presently undergoing clinical trials for various therapeutic and neuroprosthetic applications (
28). The BION (developed at the A.E. Mann Institute at the University of Southern California) is a small cylindrical (2 mm outer diameter × 16 mm length), injectable,
single-channel unit that functions as a receiver/stimulator and electrode (
29). BIONs are implanted with a 12-gauge trochar in target muscles or near target nerves; the quantity to be implanted depends on the application. An external RF coil transmits power and control information from an external control unit. Battery-powered BIONs and EMG-sensing micro-injectable units are presently under development.
These technologies and principles of electrical stimulation form the foundation for clinical applications of NMES. The next section will describe neuroprosthetic systems that have been developed for different upper motor neuron impairments.