FIGURE 2.1 Nonseptic, noninflammatory knee joint aspirate from a patient with advanced osteoarthritis.
FIGURE 2.2 Nonseptic, inflammatory knee joint aspirate with 20,365 WBCs from a patient with an acute flare of rheumatoid arthritis.
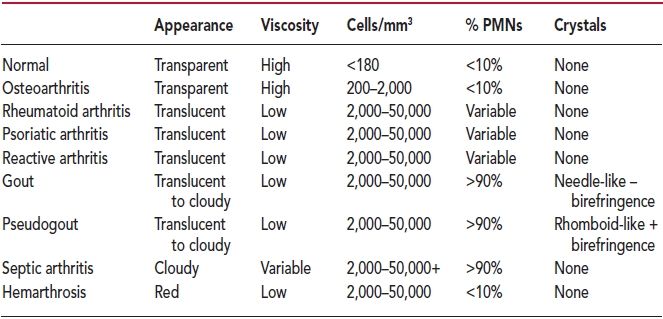
In the past, a number of tests for glucose, pH, and lactic acid were routinely performed, but evidence-based investigation has disproved their value. Traditionally, joint effusions have been classified as normal, noninflammatory, inflammatory, septic, and hemorrhagic. The absolute cell count is the major discriminating factor between an inflammatory fluid and a noninflammatory fluid. Fluids with cell counts less than 2,000 cells/mm3 are likely to be noninflammatory, and inflammatory fluids generally have more than 2,000 cells/mm3. The differential leukocyte count may add further information. Noninflammatory fluid generally contains less than 50% polymorphonuclear cells (PMNs) and an inflammatory fluid considerably more. Recent studies show that the only clinically useful synovial tests in the setting of septic arthritis are the WBC count, percentage of PMNs, Gram stain, and culture.
Crystal analysis can be performed in any office equipped with a microscope. A single drop of fluid is placed on a clean slide and is examined under a cover slip. Crystals can be observed with plain microscopy, and a preliminary identification made with regard to crystals, WBCs, and bacteria. A polarizing light microscope provides the gold standard for crystal identification. This is usually present only in a referral laboratory. Monosodium urate crystals found in gout appear needle shaped and are strongly negatively birefringent when examined under polarization. Calcium pyrophosphate dihydrate crystals are found in pseudogout. These are strongly refractile, short, rhomboid shaped, and weakly positively birefringent. The presence of intracellular crystals is an even more specific predictor for gout or pseudogout (Table 2.1).
TABLE 2.1
Synovial Fluid Properties
SPECIAL MEDICAL CONDITIONS
Several medical conditions deserve special consideration. Diabetes is a common and increasingly prevalent medical condition in the primary care physician’s patient population. Because of the frequent association with obesity, these patients place mechanical and metabolic stress on joint and soft tissues that may be amenable to treatment using therapeutic injections. Although there is concern that blood glucose levels may rise significantly, recent studies show that the transient elevation is not clinically significant. A single intra-articular steroid injection into the knee produces acute hyperglycemia for 2 to 3 days in patients with diabetes who otherwise have good glucose control.18,19 Intra-articular steroid injections into the shoulder may briefly raise postprandial (but not mean) glucose levels with larger and repeated doses.20 The rise in blood sugars can be well controlled with carbohydrate restriction, continuation of the usual diabetes treatment regimen, and close monitoring of blood sugars for a few days following injection. However, the presence of diabetes may make treatment using injected corticosteroids less effective.
Concern has been raised regarding the performance of injections in patients taking oral anticoagulants, including aspirin, NSAIDs, antiplatelet agents, warfarin, and other anticoagulant medications. There is only a single small investigation available for guidance. A prospective cohort study reported in 1998 demonstrated safe and effective joint and soft tissue injections and aspirations of 32 procedures on patients with therapeutic doses of warfarin. No episodes of hemarthrosis or abnormal bleeding were observed.8 This has also been the author’s experience in many more patients (unpublished). No studies concerning injection procedures have been conducted to date on patients receiving antiplatelet drugs, thrombolytics, fibrinolytics, low molecular weight heparin, or the newer oral anticoagulant medications, including direct thrombin inhibitors (dabigatran and others) and factor Xa inhibitors (rivaroxaban, apixaban, and others). Data on the safety of the performance of injection procedures in patients taking warfarin should not be extended to these anticoagulation agents.
Septic arthritis is a medical emergency. A joint infection is a very serious condition with dire consequences to the integrity of the joint and surrounding structures. All efforts must be made to diagnose septic arthritis as soon as possible and provide emergent hospitalization. The patient requires treatment including surgical drainage and irrigation of the affected joint, administration of intravenous antibiotics, and pain control. This is best done in a coordinated manner by the primary care physician with an orthopedic surgeon, and possibly an infectious disease subspecialist. Common organisms causing joint infections are Streptococcus/Staphylococcus species, Neisseria gonorrhoeae and, increasingly, methicillin-resistant Staphylococcus aureus.
Rheumatoid arthritis presents unique challenges to the primary care provider. This is a destructive, rapidly progressive inflammatory arthritis. Lytic enzymes rapidly degrade the joint surfaces, synovium, and supporting structures unless the process is interrupted and controlled. Joint and soft tissue injections play an important role in medical care because they can be used to deliver relatively small doses of corticosteroids locally in the affected joint(s) to augment the overall systemic management of this condition.
Management of pain involving joint replacement devices demands special consideration. Pain involving a joint replacement often occurs because the normal biomechanics are altered. Other causes of pain may include excessive postoperative scar tissue and poorly fitting or loose components of protheses. Simple injections of corticosteroids or other substances often do not lead to meaningful improvement in the patient’s pain and certainly do not correct any underlying biomechanical abnormality. There is also the possibility that an injection may be complicated by an infection of the prosthetic joint with catastrophic outcomes. In these patients, it is usually more prudent to not perform injections and to refer the patients back to their orthopedic surgeon for management of this challenging problem.
TOPICAL ANESTHESIA
Providing the patient a pain-free experience is the responsibility of the primary care provider.21 In select injections, such as the posterior approach to the subacromial space, techniques such as stretching/pinching the skin and other dermal stimulation may give adequate distraction to the patient so that pain from needle insertion is not experienced.
Painless local anesthesia via percutaneous needle introduction can be achieved by use of either skin cooling or application of a topical local anesthetic agent. A topical vapocoolant spray can be used to give rapid onset of brief, but effective, skin numbness. These skin refrigerants cause a brief period of noncytotoxic cooling of the epidermis. This provides up to 30 s of local anesthetic effect, which blocks the pain associated with needle injections. The mechanism of action for anesthesia is to decrease nerve conduction velocity of the A-delta fibers and C-fibers of the peripheral nervous system, thus interrupting nociceptive input to the spinal cord.
The classic vapocoolant agent, Gebauer’s Ethyl Chloride, is available in glass bottle and metal can containers. Both vessels are held 3 to 9 in. away from the treatment area in a well-ventilated space. The bottle is held upside down, while the Accu-Stream 360 canister may be held in any position. The stream of Ethyl Chloride is sprayed continuously to the site for 3 to 7 s from the bottle and 4 to 10 s from the can, or until the skin just turns white—whichever occurs first. The needle is then immediately inserted into the skin. Extra caution must be exercised when using Ethyl Chloride as this product is flammable. It should never be used in the setting of open flames or sparks—including cautery units, hyfrecators, electrosurgical machines, radiofrequency devices, intense pulsed light generators, or lasers.
Alternatively, Gebauer’s Pain Ease (a proprietary mixture of 1,1,1,3,3-pentafluoropropane and 1,1,1,2-tetrafluoroethane) is available both as a medium stream spray and as an aerosolized mist spray. These products are distributed in pressurized metal cans and are nonflammable products. Both Pain Ease products are administered at a distance of 3 to 7 in. from the target site by holding the can in an upright position. They are sprayed for 4 to 10 s until the skin begins to frost. Do not spray for longer than 10 s. The medium stream produces a pinpoint stream in a smaller target site. The fine droplets of mist are dispersed in a 3-cm-diameter circular pattern. Adequate local anesthesia for needle injection or minor surgical procedures lasts up to 30 s. Pain Ease is not carcinogenic or teratogenic and, thus, may be used safely in pregnancy when used as directed. Furthermore, this product offers advantages over Ethyl Chloride, including a larger field of anesthesia and lack of “running” of liquid down the skin (with use of the mist spray) and no fire hazard. In contrast to Ethyl Chloride, which is only approved for use on intact skin, Pain Ease may also be used on minor skin wounds and intact mucous membranes. With prolonged contact, both Ethyl Chloride and Pain Ease may damage polyvinylchloride coverings used to upholster examination tables. Barrier pads used during injections effectively keep the vapocoolant fluid from contact with the upholstery.
Ethyl Chloride and Pain Ease do not claim to be sterile, but the products have passed the Microbial Limit Test in accordance with the United States Pharmacopeia. These tests are designed to demonstrate that a substance is free from S. aureus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella species. Those tests also measure total bacteria, mold, and yeast growth.22 A 2012 study showed that Ethyl Chloride may be an effective disinfectant alone and may improve skin disinfection when used with povidone–iodine compared to povidone–iodine alone.23 Thus, there does not appear to be a need to wipe the field with an antiseptic agent again following the application of a topical vapocoolant spray.
LOCAL INFILTRATION ANESTHESIA
Local anesthetics are membrane-stabilizing drugs. They act by inhibiting sodium influx through sodium-specific ion channels in the neuronal cell membrane. They reversibly decrease the rate of depolarization and repolarization of excitable membranes in nociceptors—thus interrupting pain impulses.
Local anesthetics are commonly injected either alone or with another compound, such as a corticosteroid when treating painful conditions. The injection of local anesthetic into joints or soft tissues serves several purposes. Administration of the local anesthetic provides short-term pain relief. This allows for patient feedback. It may provide a more comprehensive examination of the affected area without the limitation of pain. Although mixing steroids with local anesthetics is not recommended by the manufacturers of injectable corticosteroids, it is common clinical practice to mix a local anesthetic in the same syringe as the corticosteroid solution prior to injection. By convention, the clear liquid (local anesthetic) is drawn up in the syringe first, followed by the cloudy fluid (steroid). The added volume of the local anesthetic helps dilute the corticosteroid. This enables dispersion of steroid in a large joint space or bursa. Pain relief following injection confirms the proper placement of corticosteroid both to the clinician and to the patient. Although pain may return after the effect of the anesthetic wears off, the patient can be assured that the injected corticosteroid is properly placed and should begin to exert its clinical effect within 24 to 48 h.
There are several choices of local anesthetics. Most commonly, the amino-amide class of local anesthetics is used. Lidocaine (ligocaine, Xylocaine) for local anesthetic injection is commercially available as 0.5%, 1%, and 2% concentrations with or without epinephrine. Duration of action for infiltration anesthesia is 60 to 120 min. For joint and soft tissue injections, the author exclusively uses 1% lidocaine without epinephrine. This is commonly available in multiuse bottles containing the preservative, methylparaben. Lidocaine is also available in 2-mL single-use preservative-free vials. The 2% solution of lidocaine confers no clinically important advantages and increases the risk of toxicity following administration of large amounts. The inclusion of epinephrine likewise offers no clinical advantages with musculoskeletal injections/aspirations and is not used in these procedures to dilute the corticosteroid. In fact, lidocaine with epinephrine is acidic and causes significant transient local burning pain upon injection.
Bupivacaine (Marcaine, Sensorcaine, Vivacaine, Exparel) is another commonly used local anesthetic. It has a longer onset of action but offers extended anesthetic effect. Duration of action for infiltration anesthesia is 240 to 480 min. Multidose vials also contain 1 mg of methylparaben as a preservative. Many physicians prefer to mix lidocaine with 0.25% bupivacaine in order to give the patient rapid onset of local anesthesia with an extended duration. However, there is no proven clinical benefit using this approach. Because of the additional steps required to draw up the separate anesthetics, preparation of this combination may increase the chance of contamination and needle stick injury. It may also give the patient a false sense of security since there is prolonged initial pain relief before the tissues have healed. Since the negative feedback from pain is absent for an extended period of time, the patient might suffer further injury such as tendon rupture through inadvertent use of the affected body area.
Ropivacaine (Naropin) is a newer agent that is the homolog of bupivacaine. It is available as a 0.5% solution for injection. Duration of action for infiltration anesthesia is 240 to 480 min. Levobupivacaine (Chirocaine) is the levo-enantiomer of bupivacaine. It is also a recently developed option for local anesthesia. Levobupivacaine is commercially available as 2.5, 5.0, and 7.5 mg/mL solutions. Both of these stereoisomers display less cardiovascular and central nervous system toxicity than racemic bupivacaine.24
The pH of local anesthetics can be buffered to decrease local pain. The pH of 1% lidocaine without epinephrine is 6.5 while the pH of 1% lidocaine with epinephrine is 4.5. Bupivacaine is isotonic. Adding sterile 8.4% sodium bicarbonate to lidocaine with epinephrine at a ratio of 1:10 neutralizes the mixture and has been shown to provide significant pain relief. However, this is not a clinically important issue with joint injections because plain lidocaine is used and not lidocaine with epinephrine.
A discussion of chondrocyte toxicity of amide local anesthetics is included in the “Complications” section of this chapter.
INJECTABLE AGENTS
Corticosteroids
Corticosteroids used for injection purposes are synthetic derivatives of hydrocortisone. The exact mechanism of action of corticosteroids is complex with various sites of action. They bind to glucocorticoid receptors regulating gene transcription. By altering the production of protein annexin-1, corticosteroids reduce cytokines and other inflammatory mediators.25–27 They lead to down-regulation of immune function,28 inhibition of cell-mediated immunity, and reduction in the number of macrophages and PMNs accumulating at inflammatory sites. There is also a vascular-stabilizing effect by the inhibition of endothelial expression of adhesion molecules for neutrophils. Capillary dilation and vascular permeability are therefore reduced.25,26 The end effect is to reduce the amount of inflammation, thereby reducing swelling and pain.
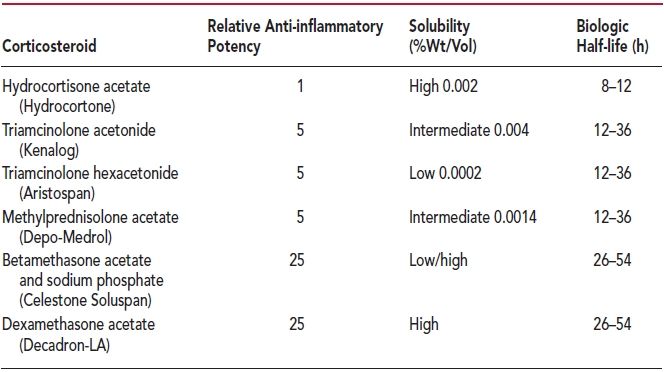
Several corticosteroids are commercially available to use for joint and soft tissue injections (Table 2.2). These include triamcinolone acetonide (Kenalog), triamcinolone diacetate (Aristocort), triamcinolone hexacetonide (Aristospan), methylprednisolone acetate (Depo-Medrol), betamethasone acetate and sodium phosphate (Celestone Soluspan), and dexamethasone acetate (Decadron-LA). The agents differ with regard to their potency, solubility, and biologic half-life (Table 2.2). Potency is measured against hydrocortisone. Different products have varying effects and solubility in the tissues. The solubility is inversely proportional to the biologic duration of effect of the agent. Hydrocortisone is almost never used because of its high solubility and very short duration of action. It also has significant mineralocorticoid activity that is not shared by the other agents.
TABLE 2.2
Properties of Injectable Corticosteroids
The common synthetic corticosteroids used in musculoskeletal procedures are derivatives of prednisolone. Corticosteroid preparations are either soluble or insoluble. Most corticosteroid preparations contain corticosteroid esters, which are highly insoluble in water and thus form microcrystalline suspensions.29 The more insoluble, esterified corticosteroids remain at the injection sites far longer than the soluble forms.
Dexamethasone preparations, however, are not esters and are freely soluble in water; hence, the preparation is clear (i.e., nonparticulate). The potential advantage of corticosteroid ester preparations is that they require hydrolysis by cellular esterases to release the active moiety and consequently should last longer in the joint than do nonester preparations.30 On the other hand, freely water-soluble preparations such as dexamethasone sodium phosphate and betamethasone sodium phosphate are taken up rapidly by cells and thus have a quicker onset of effect but with a concomitant reduced duration of action.27
Notably, the betamethasone formulation, Celestone Soluspan, contains a combination of betamethasone salt and betamethasone ester and, therefore, may provide a dual action of quick onset and long duration of therapy. However, most clinical studies have not shown a significant difference between this product and other corticosteroid ester preparations in terms of onset or duration.31,32
Few studies have been conducted that directly compare the various agents in terms of their efficacy. A comparison of intra-articular administration of triamcinolone acetonide, triamcinolone hexacetonide, and a combination of betamethasone phosphate and acetate was conducted by Derendorf and colleagues.33 They demonstrated complete absorption of all corticosteroids from the site of injection over a period of 2 to 3 weeks. Because of its lower solubility, triamcinolone hexacetonide was absorbed more slowly than triamcinolone acetonide, thus maintaining synovial levels for a longer time and creating lower systemic corticoid levels. Endogenous hydrocortisone suppression correlated with exogenous steroid levels.33
No studies have been done that conclusively determine which corticosteroid is preferred for injection of joints or soft tissues. Without good data, the selection of the particular corticosteroid agent is left to the preference of the individual clinician. Despite lack of literature support, some clinicians prefer to choose a relatively insoluble preparation for intra-articular use and a more soluble form for use in soft tissues and peritendinous injections. Considering medication availability, cost, and past experience, the author prefers to use triamcinolone acetonide (40 mg/mL) for all injections, regardless of site. If another corticosteroid is chosen, then the equivalent dosage and volume of administration may be calculated from the comparison table (Table 2.3).
TABLE 2.3
Equivalent Dosages of Injectable Corticosteroids
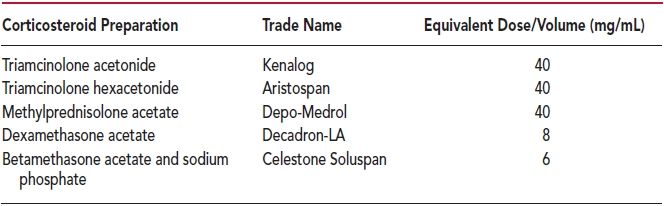
The dose of corticosteroid to be used generally depends on the injection site, disease process, and degree of inflammation. Suggested doses of corticosteroid are listed in each individual chapter. Table 2.3 presents equivalent dosages of corticosteroids used for injection. For the purpose of this book, all doses are expressed in milligrams of triamcinolone acetonide suspension (Kenalog). If the physician chooses to use another steroid, then the comparative dosage can be simply calculated from the table. For instance, if the chapter in this text indicates that 20 mg of triamcinolone is to be used for injection into the wrist joint, then one could use 20 mg of Kenalog, 20 mg of Aristospan, 20 mg of Depo-Medrol, 4 mg of Decadron-LA, or 3 mg of Celestone Soluspan.
All published information concerning the frequency of intra-articular corticosteroid injection appears to be based upon professional opinion. A search of the peer-reviewed published medical literature failed to identify any studies that investigate how often corticosteroids can be injected into a joint. In general, most experts advocate that corticosteroid injections should be performed no more often than every 3 months. This non–evidence-based guide is an attempt to prevent potential steroid-related complications, including hypothalamic–pituitary–adrenal axis suppression, osteoporosis, and local articular degradation.
The author typically uses two syringe sizes when injecting corticosteroids. A 3-mL syringe is used for most injection sites of small joints, medium joints, and soft tissues. This accommodates 1 mL of 1% lidocaine without epinephrine and 1 mL of corticosteroid. A 5-mL syringe is used for large joints and bursa such as the subacromial space, sacroiliac joint, hip joint, knee joint, and trochanteric bursa. Each syringe is prepared at the time of the procedure. In the case of a large joint, 3 mL of 1% lidocaine is drawn up in the 5-mL syringe and then followed by drawing up 1 mL of the corticosteroid. Prior to injecting a patient with the local anesthetic/corticosteroid mixture, a common observation is that the insoluble corticosteroid often precipitates along the dependent aspect of the syringe. Immediately before the local anesthetic/corticosteroid mixture is injected, 1 mL of air is aspirated into the syringe creating a “mixing bubble” (Fig. 2.3). The syringe is then rapidly rotated in order to disperse the corticosteroid evenly within the volume of local anesthetic. The needle of the syringe is then pointed upward and the small volume of air expelled before the needle is inserted into the skin at the target site.
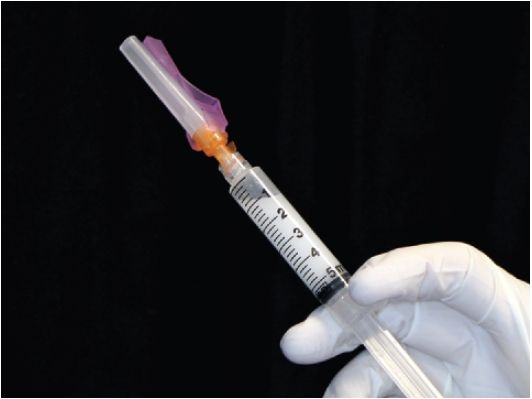
FIGURE 2.3 Mixing bubble.
There is a common misconception that distributing the corticosteroid over a wide area enhances the effect from soft tissue injections. Practitioners frequently use a “fanning” or “peppering” technique to distribute the solution across the area of involvement. However, this practice is usually unnecessary. Solution injected as a bolus will passively move along tendon sheaths and local fascia planes. Consideration might be given to “fanning” when injecting trochanteric bursitis because the involved area is frequently quite large.
Viscosupplementation
Hyaluronan (sodium hyaluronate) is a natural complex sugar of the glycosaminoglycan family. The concentration and size of endogenous hyaluronan are reduced in the joint fluid of patients with osteoarthritis. Currently, there are several products available for injection that can be used to supplement this substance in joint fluid. These commercial agents are high molecular weight derivatives of hyaluronan, which are synthetically derived from rooster combs or produced by bacterial fermentation and extraction. The exact mechanism of action of viscosupplements is unknown but may involve physical cushioning of the knee joint, anti-inflammatory action, and/or the stimulation of production of endogenous hyaluronan by synoviocytes.
Viscosupplements serve an important role in the treatment of osteoarthritis of the knee. This condition is a chronic disease state that has a number of therapeutic options. Of these, weight reduction and physical therapy are the most effective measures. However, when pain persists, pharmacologic options may be utilized. Unfortunately, oral NSAIDs and acetaminophen are only modestly effective and possess significant toxicity. Narcotic medications should not be used on a chronic basis in this condition. Corticosteroid injections are effective for short- to medium-term use especially in patients experiencing an acute flare in pain and swelling. Viscosupplements occupy an important position in the mid- (3 months) to long-term (6 months) treatment of knee osteoarthritis. Their use may allow appropriate postponement of knee replacement surgery. They have an excellent record of effectiveness and long-term safety.
Injectable hyaluronan is commercially available in the United States as the products Synvisc (Genzyme), Synvisc-One (Genzyme), Gel-One (Zimmer), Orthovisc (Depuy Mitek), Hyalgan (Fidia Pharma), Supartz (Bioventus), and Euflexxa (Ferring) (Table 2.4). They are classified not as medications, but actually as medical devices by the FDA. These agents are approved only for the treatment of pain in osteoarthritis of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics such as acetaminophen. The safety and effectiveness of the use of these viscosupplements in other joints have not been established.
TABLE 2.4
Viscosupplementation Products






