Fixation Using Bone Substitutes
Amy L. Ladd
Arthur T. Lee
Introduction
An extensive variety of graft substitutes serve as gap fillers that can aid in restoration of structural integrity for voids and fracture fixation; the common fracture of the distal radius has been a natural target for product design and development (1,2,3). Only relatively recently were distal radius fractures treated with grafting: Improved external and internal fixation techniques in conjunction with autogenous bone grafting were reported in the 1980s and 1990s, and thus such is recognized as an important adjunct (4,5,6). The benefits of autogenous graft included faster healing, earlier removal of external fixation, and improved anatomic and functional outcome. The benefits, however, accompany morbidity and hospitalization costs specifically with iliac crest graft harvest (Table 10-1) (7). This disparity influenced surgeons and entrepreneurs alike to seek better alternatives for fracture augmentation, and numerous substitutes have been in use throughout the musculoskeletal system, in addition to the distal radius. The indications and potential indications for current use of substitutes in metaphyseal voids and fractures about the wrist are the primary focus of this chapter. The use of organic and polymeric grafts still in development or not widely used are beyond the scope herein, but many examples may be found in Tables 10-2, 10-3, 10-4, 10-5 and 10-6.
Mineral Substitutes Commonly Used in the Distal Radius
Ceramics and Cement
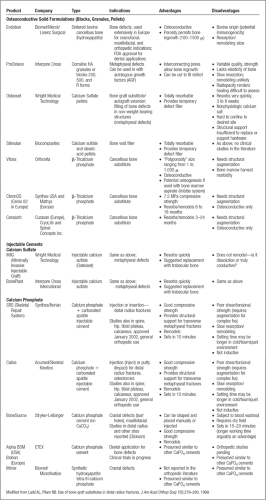
The mineral substitutes may arbitrarily be divided into ceramics and cements (Tables 10-3 and 10-4). Ceramic is a mineral salt heated to high temperatures, greater than 1,000ºC, in the process known as sintering. Sintering provides strength to a finished material, but when applied to bone or similar minerals, decreases the remodeling and resorption capability of bone.
Many surgeons express concern about the slow disappearance, whether by resorption or remodeling, of the mineral substances on radiographs. This concern is supported by several arguments: The material is a foreign object, it obscures bone healing, and it potentially alters the mechanics of joints and soft tissue, because this is a harder substance than the surrounding bone. Although this concern may be unsubstantiated, radiographic appearance of bone trabeculation replacing the substitute is an increasingly desirable attribute of new materials. More extensive interconnected porosity of a substance permits faster bony ingrowth but weakens the material; the ideal pore size is thought to be between 150 and 500 μ. Both sintered and nonsintered porous substances exist, the latter of which permit faster resorption. Whether resorption correlates with remodeling depends on the material and the local environment.
Coral
Coralline HA is coral that is thermochemically treated with ammonium phosphate, which demonstrates porosity similar to bone (Fig. 10-1). The thermochemical conversion process for Pro Osteon (Interpore Cross International, Irvine, California, now a Biomet company) converts 95% of the coral’s calcium carbonate into more slowly resorbed HA. This is not a sintering process, and it is therefore not technically a ceramic, but the conversion has a similar effect of minimizing its resorption. Bone ingrowth has been demonstrated in the interconnected pores, with osteoblasts evident on the surface of the material (14). Remodeling has not been identified. Formulations include different pore sizes, with the larger (approximately 500 μ) pore size more appropriate for cancellous
bone defects. Its compressive strength is reported as approximately 4 MPa. The 200-μ size is used more commonly in dentistry. Recent formulation with calcium carbonate on its surface (Pro Osteon R) permits some resorption, and potentially better ingrowth. Pro Osteon was the first calcium phosphate-based bone graft substitute to receive FDA approval for fracture treatment in 1992, and received general use approval in 1998. The 500-μ pore size formulation has found widespread orthopedic applications, and is shown to be useful in fractures of the distal radius for both internal and external fixation (15).
bone defects. Its compressive strength is reported as approximately 4 MPa. The 200-μ size is used more commonly in dentistry. Recent formulation with calcium carbonate on its surface (Pro Osteon R) permits some resorption, and potentially better ingrowth. Pro Osteon was the first calcium phosphate-based bone graft substitute to receive FDA approval for fracture treatment in 1992, and received general use approval in 1998. The 500-μ pore size formulation has found widespread orthopedic applications, and is shown to be useful in fractures of the distal radius for both internal and external fixation (15).
Table 10-1. Advantages and Disadvantages of Autologous Bone Graft | ||||
|---|---|---|---|---|
|
Table 10-2. Human Allograft Bone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 10-3. Mineral Composites | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Osteoinduction may be introduced with the osteoconduction of the coralline HA when grafting is combined with autologous platelets from a special autotransfusion process. The cytokines transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF) have been identified in this filtrate through the technique called Autologous Growth Factors (AGF, also marketed by Interpore Cross; see platelet rich plasma (PRPs) in Proteins and Growth Factors, Table 10-6).
Calcium Sulfate
Calcium sulfate, better known as plaster of paris, results from the calcination of gypsum (CaSO4, 2 H2O), which partially dehydrates to produce a hemihydrate (CaSO4, 1/2 H2O) (Fig. 10-2). Because the clinical use of calcium sulfate predated the existence of the FDA, it was designated a class II “special controls” device in 1998, requiring institution of voluntary consensus standards for its use. This consensus is known as surgical grade, reflecting purity and consistency of the material. The first calcium sulfate marketed was Osteoset (Wright Medical, Arlington, TN), available in pellet form, and more recently as an injectable (Minimally Invasive Injectable Graft, MIIG); see Table 10-4 for other formulations.
Table 10-5. Bioglasses and Polymers | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Table 10-6. Factors and Proteins | |||||
|---|---|---|---|---|---|
|