Chapter 166 Fibromyalgia Syndrome
 Diagnostic Summary
Diagnostic Summary
• Common: 2% to 13% of population; approximately 80% to 90% women
• Chronic widespread pain involving axial pain and pain on the left, right, upper, and lower parts of body1
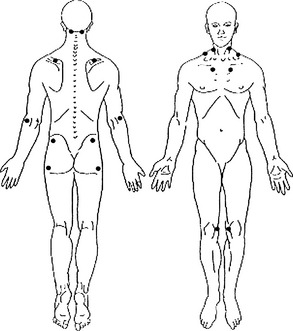
• Abnormal tenderness at 11 or more of 18 specific anatomic tender point (TnP) sites1 (Figure 166-1)
 Diagnosis
Diagnosis
The 13 most common symptoms associated with the pain and tenderness of fibromyalgia syndrome (FMS) 2–4 are listed in Box 166-1.
Differential Diagnosis
The purpose of the 1990 American College of Rheumatology (ACR) criteria for FMS was to distinguish patients with a putative primary disorder designated FMS from those with similar symptoms due to other distinguishable medical disorders. The two major criteria for FMS are chronic (longer than 3 months) widespread pain and tenderness.1 The criteria were established mainly for use in research that would eventually identify the underlying pathologic mechanism of the symptoms, but they have come to serve as diagnostic criteria in clinical practice.
Some theorists have argued that widespread pain and tenderness at predictable anatomic sites are features of many medical disorders.5 Their argument is true only of hypothyroidism and cellular resistance to thyroid hormone, especially when the associated symptoms of FMS are also taken into account. When most patients with hypothyroidism or cellular resistance to thyroid hormone meet the ACR criteria for FMS and are effectively treated with thyroid hormone therapy, they no longer meet the FMS criteria.6–15 This finding indicates that these patients’ FMS symptoms and signs are a distinct clinical phenotype of inadequate thyroid hormone tissue regulation.16 It also justifies a trial of thyroid hormone therapy to distinguish whether a patient’s FMS symptoms and signs are features of hypothyroidism or cellular resistance to thyroid hormone.
The theorists’ argument does not apply to other medical disorders, however, and is refuted by two lines of evidence.17 First, studies have not established that chronic widespread pain and multiple TnPs are features of other medical disorders. Second, many patients with rheumatoid arthritis, osteoarthritis, Lyme disease, systemic lupus erythematosus, Chiari malformation, and spinal stenosis also meet the ACR criteria for FMS, but when these other disorders are effectively treated, the patients still meet the criteria for FMS.18–20
The argument is false, then, that chronic widespread pain and tenderness are features of many medical disorders. Nonetheless, the argument highlights the need to differentiate FMS from other disorders with similar symptoms that may lead the clinician to misdiagnose FMS. The main disorders the clinician should distinguish from FMS are arthritis, myopathy, polymyalgia rheumatica, diabetic polyneuropathy, ankylosing spondylitis, discopathy, cardiac or pleural pain,21 multiple muscle myofascial pain syndromes,22 and lupus erythematosus. A given patient may, of course, concurrently meet the criteria for FMS and one or more other disorders with overlapping symptoms. FMS can usually be distinguished from medical disorders other than hypothyroidism and cellular resistance to thyroid hormone by careful pathognomy. However, diagnostic scrutiny will show that the symptoms and signs of most FMS patients are indistinguishable from those of the subclass of hypothyroid and thyroid hormone–resistant patients for whom pain is a predominant symptom.
 General Considerations
General Considerations
Current Focus of the Conventional Fibromyalgia Research Community
The serotonin deficiency hypothesis is the oldest proposed mechanism of FMS.23–25 As a research-inspiring concept, it constituted the core theoretical underpinning of the rheumatology paradigm of FMS. This hypothesis proposed that a central nervous system (CNS) serotonin deficiency reduced the efficiency of the brainstem–spinal cord descending antinociceptive system, lowering the threshold for pain perception.26 By 2000, the serotonin deficiency hypothesis had been effectively refuted16 by three lines of evidence: First, the only site of low serotonin among FMS patients was their platelets.26–28 Second, serotonin-increasing drugs were no more effective than placebos29,30–34 and exacerbated some patients’ FMS status.35 And third, the low brain blood flow of FMS patients36,37 contradicts a serotonin deficiency; serotonin is a potent vasoconstrictor,38 and a low CNS level would produce cerebral vasodilation and increased blood flow.
This was further documented in the 2005 publication of the state of that paradigm.39 The central theoretical viewpoint of the former paradigm had shifted from a serotonin deficiency to a generalized problem of augmented pain processing resulting in a hyperalgesic state,39,40 which is plausibly explained by the extraordinarily high levels of substance P in patients with FMS.41 But researchers espousing the former paradigm have failed to address the most plausible mechanism of the patients’ high levels of substance P (see following section). Instead, their main efforts have been to develop pharmaceuticals to manage FMS pain. This has led to the approval of several drugs by the U.S. Food and Drug Administration for FMS and the recommendation that patients undergo education about FMS as well as psychotherapy and aerobic, strength, and flexibility training.40
Hypometabolism Hypothesis of Fibromyalgia Syndrome
The hypometabolism hypothesis posits that FMS is chronic hyperalgesia and other symptoms and signs of hypometabolism due to hypothyroidism, partial cellular resistance to thyroid hormone, or other metabolism-impeding factors. Among the other factors said to be responsible are pernicious diet, nutritional deficiencies, low physical fitness, and metabolism-impeding drugs. The term hypometabolism refers to the global impact on the patient of the underlying factors, most of which are catabolic and inhibitory, although some are anabolic or excitatory.16
• Tenderness (lowering of the pain threshold to mechanical stimuli)
• Hyperalgesia (increased responsiveness to noxious stimuli)43,44
ITHR can impair the antinociceptive system by two mechanisms. First, ITHR can severely increase production of substance P, which is extremely high in the cerebrospinal fluid (CSF) of tested FMS patients.41 Substance P is released from the terminals of nociceptive neurons42 and assists the summation of slow nociceptive signals.45 This facilitation amplifies the transmission of nociceptive signals in the spinal cord.46 Thyroid hormone normally inhibits the synthesis and secretion of substance P in many CNS cells. It does so by repressing the transcription of the preprotachykinin-A gene. Preprotachykinin-A is the precursor of substance P and its cognate substance P receptor.47,48 Lowering thyroid hormone levels by thyroidectomy increased the substance P level in astrocytes48; anterior pituitary49–51; many brain nuclei52; and, most relevant to pain, the dorsal horns of the lumbar spinal cord. The increase in dorsal horn substance P was highly elevated (100%),53,54 as in FMS patients.41 Thyroid hormone treatment lowered the substance P level in the anterior pituitary,50 brain nuclei,55 and dorsal horns.54 Excess thyroid hormone reduced substance P to subnormal levels.51
The second mechanism by which ITHR can reduce the effectiveness of the antinociceptive system is by reducing the synthesis and secretion of norepinephrine (NE) in cells of the brainstem locus ceruleus. Adequate NE is essential to normal function of the descending antinociceptive system.56,57
The antinociceptive pathways that descend from the brainstem to the dorsal horns contain two types of neurons: those that secrete serotonin and others that secrete NE.20,56,58,59 Serotonin secretion by the neurons is tonically augmented by NE secretion. Normal serotonin secretion is therefore dependent on NE secretion.54 The serotonin stimulates interneurons to secrete opiates.56,58,60 These then inhibit transmission partly by blocking release of neurotransmitter substances such as glutamate and substance P from the afferent neurons.61 They also block calcium influx and potassium efflux from the afferent terminals,62 mainly those of types C and A delta fibers.63 The decreased potassium efflux hyperpolarizes terminals, inhibiting the transmission of nociceptive signals to spinothalamic neurons that otherwise would transmit the signals to the brain.64 Low NE secretion by descending neurons, however, may reduce the secretion of serotonin selectively at dorsal horn interneurons and secondarily reduce opiate secretion. As a result, the transmission of nociceptive signals in the CNS will increase, thus heightening pain perception.
That decreased NE production is involved in the heightened pain perception of FMS patients is indicated by low metabolites of both dopamine and NE in patients’ CSF.27 That inadequate T3 regulation of locus ceruleus neurons accounts for the low NE production is suggested by the crucial role T3 plays in the synthesis of both dopamine and NE. The locus ceruleus is the brain site with the heaviest concentration of T3.65–67 Thyroid hormone regulates the activity levels of two rate-limiting enzymes in dopamine and NE synthesis.68 One enzyme, tyrosine hydroxylase, catalyzes the conversion of tyrosine to levodopa, which in turn is converted to dopamine.69 Tyrosine hydroxylase activity in the noradrenergic neurons of the locus ceruleus is low in hypothyroidism.70 Low activity of the enzyme and reduced conversion of levodopa to dopamine may be responsible for low dopamine levels in the striatum, hypothalamus, and superior cervical ganglia in hypothyroidism.68 Thyroid hormone therapy increases the activity of tyrosine hydroxylase.70 The second enzyme, dopamine-β-hydroxylase, catalyzes the conversion of dopamine to NE. Low activity of the enzyme in hypothyroidism can reduce NE levels.68 Unfortunately, NE levels in the antinociceptive system and other tissues in thyroid disorders have not been studied extensively71 enough to support this putative mechanism.
By raising substance P levels and possibly lowering NE levels in the spinal cord, ITHR can thus plausibly heighten FMS patients’ pain perception. Patients’ pain, however, is probably compounded by other factors. First, most FMS patients are physically inactive because of their pain,72,73 although low motor drive from low dopamine levels27 probably contributes to their inactivity. Their low physical activity level may further contribute to the inefficiency of the antinociceptive system.74–76
ITHR can also plausibly account for the other symptoms and objectively verified abnormalities of FMS: muscle and joint pain, paresthesias, cognitive dysfunction, depression, cold intolerance, exercise intolerance, weakness and fatigue, dry skin and mucous membranes, constipation, dysmenorrhea, and menorrhagia,77,78 increased platelet α2-adrenergic receptor density,79,80 reduced brain blood flow,81 reduced peripheral blood flow,82 sleep disturbance,83 deficient slow-wave sleep,84 hypotension,85 blunted sympathetic response to stress,86 stiffness and swelling,87 irritable bowel syndrome,88 excessive urination,83 high serum hyaluronic acid,89 low procollagen III,90 high ground substance proteoglycans,91 low pyridinoline92 and hydroxyproline,93 glycolysis abnormalities,94 low concentrations of high-energy phosphates in erythrocytes and muscle cells,95,96 and low growth hormone and somatomedin C levels.97 The cellular and genomic actions of thyroid hormone can explain the hormone’s relationship to all of these factors.16 Especially important is thyroid hormone’s effect on the adrenergic system. If the hypometabolism hypothesis is verified at some point in the future, the definition of fibromyalgia will include an explanation of FMS as a condition of α-adrenergic dominance.
Experimental Support for the Hypometabolism Hypothesis of Fibromyalgia Syndrome
The hypothesis that ITHR is an underlying mechanism of FMS has considerable experimental support.98 Many researchers have noted the virtually identical features of FMS, hypothyroidism, and the peripheral form of cellular resistance to thyroid hormone.16,99–114
Several research groups have used thyroid function testing to determine the incidence of thyroid disease among FMS patients. Each group has reported an incidence higher than in the general population.99–109 A thorough analysis of all the available evidence indicates that approximately 90% of FMS patients have some form of thyroid disease, either primary or central hypothyroidism or cellular resistance to thyroid hormone.115,116
In two studies, 15 female FMS patients had low resting metabolic rates (RMRs) and basal axillary temperatures compared with 15 matched healthy controls. In both studies, the controls’ mean RMR was within the reference range predicted by equations. However, in the first study, the mean RMR of FMS patients was 29.2% below normal based on their gender, age, height, and weight.117 In the second study, FMS patients’ mean RMR was 32.5% below normal.118 In both studies, FMS patients’ basal temperatures were significantly lower than those of controls. In the first study,117 FMS patients’ mean basal temperature was 96.95° F (36.08° C). In the second study,118 the mean temperature of FMS patients was 96.38° F (35.77° C).
The only clinical trials in which patients have been fully relieved of FMS have involved orally administered thyroid hormone. It should be noted, however, that in each study patients also used other metabolism-regulating therapies (see later discussion on metabolism-regulating therapies other than thyroid hormone). Euthyroid and hypothyroid FMS patients fully recovered in five open but highly systematic trials,6–10 three double-blind placebo-controlled crossover trials,11–13 and a randomized double-blind placebo-controlled trial.14 In another randomized double-blind trial, FMS patients had limited improvement with the use of transdermal T3.119 A 1- to 5-year follow-up study compared the status of control FMS patients with FMS patients who had either hypothyroidism or cellular resistance to thyroid hormone and underwent metabolic therapy, including the use of thyroid hormone. Although control patients’ FMS status deteriorated, treated patients recovered and maintained their recovery throughout the follow-up period. Results of this study were the first to demonstrate the long-term effectiveness of an FMS treatment.15
Metabolism-Regulating Therapies Other Than Thyroid Hormone
Thyroid hormone therapy is necessary in most cases of FMS to ensure recovery, but it is not sufficient. For example, in a study of 77 euthyroid FMS patients,6 those who declined to adopt a wholesome diet, take nutritional supplements, and exercise to tolerance were among the 25% who failed to benefit from T3 therapy. This result is consistent with years of clinical experience supporting the necessity for patients to use other metabolism-regulating therapies in addition to thyroid hormone. A common experience, for example, is a recovered patient stopping her use of B complex vitamins only to suffer a partial recurrence of FMS symptoms. The symptoms are again relieved when she resumes her use of the vitamins.
Wholesome Diet
Various diets and dietary supplements have been tested as FMS treatments. Mild-to-moderate improvements in FMS status have been reported for vegetarian diets120,121; elimination of the excitotoxins monosodium glutamate and aspartame122; Chlorella pyrenoidosa (a unicellular freshwater green alga rich in proteins, vitamins, and minerals)123,124; an uncooked vegan diet consisting of berries, fruits, vegetables and roots, nuts, and germinated seeds and sprouts125; and a strict, low-salt, uncooked vegan diet rich in lactobacteria.126
Nutritional Therapies
Studies indicate that vitamins B1, B6, B12,56,127–135 C,136–140 and E, and beta-carotene141 have antinociceptive properties. Other reports indicate that various nutritional supplements improve FMS status: the Myers’ cocktail (intravenous formula of B vitamins, vitamin C, calcium, and magnesium)142; 5-hydroxytryptophan143,144; S-adenosylmethionine145–148; magnesium and malic acid149,150; combined aloe vera extracts, plant saccharides, freeze-dried fruits and vegetables, Dioscorea, and a vitamin/mineral complex151; collagen hydrolysat152; and a blend of ascorbigen and broccoli powder.153
Exercise to Tolerance
The results of studies of exercise in the treatment of FMS have been mixed: some have shown no improvement in FMS status35,69,154,155–157 or only minimal improvement.158–163 Cardiovascular exercise has provided the most improvement,160–163 especially low-intensity endurance training.163,164 Endurance exercise is important in reducing the physical limitations associated with FMS.165 The importance of physical fitness and the high metabolic efficiency it provides166,167 was shown by deficient slow-wave sleep, inducing FMS-like symptoms in sedentary and not aerobically fit students.23 This finding suggests that low metabolic efficiency due to ITHR renders some individuals susceptible to FMS.16
Vigorous exercise tends to exacerbate patients’ FMS symptoms.72,73 As a result, some patients avoid exercise altogether and consequently are not physically fit.165,168–170 The most plausible explanation for the exacerbations on vigorous exercise is the high-density of α2-adrenergic receptors on FMS patients’ platelets.171 Platelet density of α2-adrenergic receptors is a reliable indicator of the receptor density in the CNS.172 Binding of catecholamines to a high density of the receptors on cells of most tissues inhibits energy metabolism. The inhibition of energy metabolism during vigorous exercise, mediated by the high density of the receptors, appears to worsen symptoms severely. During the early phase of treatment, then, exercise should be mild enough to avoid or minimize catecholamine secretion.16 With effective thyroid hormone therapy, the density of α2-adrenergic receptors decreases and the density of β-adrenergic receptors increases, enabling cells to respond appropriately to high levels of catecholamines. The shift away from α2-adrenergic receptor dominance probably explains in part the FMS patient’s ability to engage in vigorous physical activity after receiving thyroid hormone therapy.6,7,8,11–13,15
Physical Treatment
Extensive clinical experience and clinical trials7,8,11–13 show that despite the use of integrated metabolic therapies, many patients require physical treatment to fully relieve their FMS pain.173–176 Several studies have shown that spinal manipulation, soft tissue manipulation, and trigger point therapy provide palliative improvement in some FMS symptoms, especially pain.177–180
The most common lesions that exacerbate FMS symptoms are myofascial trigger points and spinal joint fixations.16,174,181 However, any nociception-generating neuromusculoskeletal lesion may exacerbate FMS patients’ pain, probably owing to their high levels of substance P.40 Neuromusculoskeletal lesions can also disturb sleep.182–184 This can, in turn, increase FMS symptoms.23
 Therapeutic Considerations
Therapeutic Considerations
Laboratory Testing for Thyroid Status
Thyroid Function Tests
In untreated primary hypothyroidism, the TSH is elevated. The recently revised upper limit of the serum TSH reference range is 2.5 µIU/mL.185
Thyroid Antibodies
The most common cause of primary hypothyroidism is autoimmune thyroiditis. The presence of this disorder can be determined by the patient’s titer of thyroglobulin and thyroid peroxidase (microsomal) antibodies. It is especially important to order tests of antibody levels in FMS patients. Many patients who have elevated thyroid antibodies have also had thyroid function test results within the reference ranges for many years.187,188 But compared with people without chronic, widespread musculoskeletal complaints, those with such complaints were found to have a significantly higher incidence of thyroid microsomal antibodies (16% vs. 7.3%, P <0.01). The prevalence of antibodies was also significantly higher in women than men (20.4% vs. 11.6%, P <0.02). However, thyroid function test results did not differ significantly between those with and without musculoskeletal complaints.119 This study indicates that in patients with autoimmune thyroiditis, thyroid hormone levels too low to properly regulate the CNS antinociceptive system and properly inhibit the production of substance P can escape detection by thyroid function tests, including the TSH.
Thyroid Hormone Therapy Based on Initial Thyroid Status
FMS patients whose test results indicate hypothyroidism should begin therapy with a thyroid hormone preparation containing both T4 and T3. Many hypothyroid FMS patients do not benefit from T4 alone no matter how high the dosage.189 Most do benefit, however, from the use of T4/T3 preparations in a 4:1 ratio. The dosage range at which most patients improve or recover with T4/T3 preparations is that which was used throughout the twentieth century without harmful effects190 before TSH assays came into widespread use in the early 1970s: 2 to 4 grains (76 mg T4 and 18 mg T3 to 152 mg T4 and 36 mg T3).16,191
The thyroid hormone therapy used in studies in which hypothyroid and euthyroid FMS patients recovered6–14 was not conventional T4-replacement therapy. Although T4 was used in some studies,9,10,14,15 it was T4/T3 preparations and T3 alone used in a way that violated the mandates of T4 replacement. Specifically, patients were permitted to use thyroid hormone despite laboratory test results indicating that they were euthyroid, and doses were not titrated according to TSH levels but by patients’ clinical responses to particular doses.
FMS patients whose laboratory test results indicate euthyroidism should begin with T3. In studies of the thyroid status of FMS patients at intake, approximately 33% were euthyroid according to thyroid function testing.186,192 Of these patients, approximately 75% have partial peripheral cellular resistance to thyroid hormone according to four criteria115,116:
1. The patients are euthyroid (according to laboratory thyroid function test results) before beginning the use of T3.
2. The patients recover from their hypothyroid-like FMS symptoms and signs with supraphysiologic doses of T3.
3. The patients have high serum-free T3 levels after beginning T3 therapy.
4. The patients have no evidence of tissue thyrotoxicosis, according to the results of serial ECGs, serum and urine biochemical tests, and bone densitometry.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree