Femoral Head Sparing Procedures for Osteonecrosis of the Hip
Michael A. Mont, Michael G. Zywiel and Edward H. Becker
Key Points
Introduction
Multiple treatment options are available for osteonecrosis of the femoral head. If the disease has progressed to severe femoral head collapse and/or acetabular damage, only a hip arthroplasty will markedly relieve pain and improve function. However, for lesions that are not as advanced, multiple joint-preserving treatments have been successfully utilized to improve symptoms and delay or prevent arthroplasty.
Early diagnosis and accurate staging of the disease are important for selecting the optimal treatment. Different treatment modalities are indicated for different stages of the disease, and overall success rates are higher at earlier disease stages. Therefore, it is important to understand how to make the earliest possible diagnosis based on history and physical examination and how to plan treatment based mostly on radiographic indices.
To make an early diagnosis, the clinician should understand that osteonecrosis is frequently associated with one or more risk factors such as corticosteroid or excessive alcohol use (Box 58-1), which, if present in combination with symptoms, should trigger a low threshold for radiographic evaluation. The most common clinical symptoms of osteonecrosis include deep, throbbing groin pain and limited range of motion, especially of internal rotation.1 Pain may be localized to the buttock, thigh, or knee. It often occurs with hip movement or weight-bearing activities, although rest pain is noted in more advanced disease. Pain usually has a gradual onset, although acute onset of symptoms may occur. Some patients have minimal pain; others are asymptomatic, irrespective of radiographic appearance.
Radiographically, osteonecrosis has a wide range of findings, and many different classification systems have been used to characterize this disease and to help stage it for diagnosis, treatment, and prognosis. For example, in one recent report, more than 23 classification systems were cited. Fortunately, most publications have described one of five commonly used systems (Table 58-1).2–6 However, the present authors have found it more useful to understand four primary radiographic factors in staging and planning the most appropriate head-sparing procedure for these patients. These radiographic features include (1) precollapse or postcollapse (presence of crescent sign) femoral heads, with precollapse lesions having the best prognosis, (2) size of the lesion, with larger lesions having a worse prognosis than small or medium-sized lesions, (3) amount of femoral head depression, with greater than 2 mm appearing to be the cutoff for not using head-preserving procedures, and (4) acetabular involvement with disease, in which head-preserving procedures should not be used. Some authors have characterized the location of the lesion as important in determining prognosis and a treatment plan, as outlined by the Japanese Orthopaedic Association. For example, lateral lesions have a worse prognosis than medial lesions, and central lesions are more intermediate. The present authors have found that location sometimes adds little prognostic information to the size evaluation because most lateral lesions are large, and medially located lesions are usually small.
Table 58-1
Overview of Various Staging Systems for Osteonecrosis of the Femoral Head
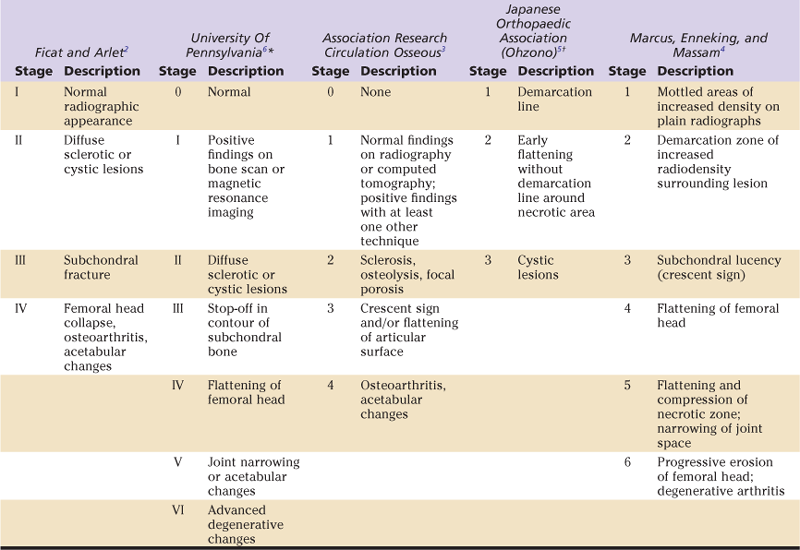
*Further stratified as A,B, or C, depending on severity.
†Stratified by medial to lateral weight-bearing area involvement.
In the following description of various head-preserving procedures, we will discuss where different procedures are used based on these four radiographic features. In addition, if the clinician is reviewing the literature, one can easily convert these features into different classification systems.
To analyze these radiographic features, it is necessary only to get good quality anteroposterior and frog-leg lateral radiographs, as well as magnetic resonance imaging (MRI) scans. When the disease is obvious (e.g., postcollapse disease with a crescent sign), it may not even be necessary to obtain an MRI evaluation. However, MRI examinations are more than 99% sensitive and specific for the disease, and rapid low-cost screening protocols have been developed that take approximately 15 minutes to perform. Other screening tests such as bone scans, computed tomography scans, and bone biopsies, although of historical importance, are not necessary for the diagnosis of osteonecrosis. For example, in one recent study, bone scanning missed 44% of symptomatic osteonecrotic lesions in 48 patients who were otherwise diagnosed with MRI.7 Computed tomography scanning is not recommended because it exposes patients to unnecessary radiation risks.
Once osteonecrosis has been diagnosed and characterized radiographically, a treatment plan can be proposed. The radiographic evaluation, in the authors’ opinion, is of paramount importance over other demographic factors in formulating this strategy. However, one may occasionally consider various demographic factors such as patient age or morbidity in deciding on a procedure. For example, one would be more likely to try a head-preserving procedure in an 18-year-old patient versus a 62-year-old patient who might be served better with a hip arthroplasty procedure. In addition, patients who have many comorbidities may not want to risk a procedure with a higher risk of failure necessitating reoperation, and may opt for a more definitive procedure. With these considerations in mind, the various nonarthroplasty procedures that have been used to treat osteonecrosis of the femoral head will be discussed, including indications and contraindications for each procedure, the surgical technique and relevant variations, postoperative care, results, and complications.
Core Decompression and Percutaneous Drilling
Core decompression is a surgical technique that involves removing a cylindrical core of bone from the proximal femur, creating a tract that extends from the subchondral region of the femoral head to the extraosseous space adjacent to the lateral proximal femur. This technique was originally described by Ficat and Arlet8 and by Hungerford9 as a method of diagnosis by histologic examination of removed bone and as a method of pain reduction. This procedure is no longer necessary for the diagnosis of osteonecrosis because magnetic resonance imaging is currently the preferred modality.10,11 Core decompression continues to be used for pain reduction and to slow the progression of disease, although some controversy continues regarding the degree to which the procedure influences the natural course of this disease. It is believed that bone removal may reduce intramedullary pressure caused by various factors such as increased adipocyte density or venous congestion within the bone, which are associated with osteonecrosis.12 Multiple animal and human studies have reported neovascularization and improved osseous blood flow following core decompression.13,14 This has been described as a simple, low-morbidity method of treating osteonecrosis, although some studies have reported complication rates of 10% or higher.
Indications and Contraindications
Core decompression can be used in patients who have precollapse osteonecrosis of the femoral head. Success rates are highest in patients who have Ficat and Arlet stage I disease (changes visible on magnetic resonance imaging only) and in those who have smaller lesions encompassing less than 25% of the volume of the femoral head. However, the procedure has been demonstrated to slow or arrest progression in Ficat and Arlet stage II and III disease, albeit less frequently. Because of the minimally invasive nature of the procedure and the low associated morbidity, especially when percutaneous drilling techniques are used, core decompression may be an excellent first choice of surgical treatment for osteonecrosis in many patients with precollapse disease. It should not be used for patients with advanced postcollapse disease or very large stage II and III lesions that have a high risk of progression or have already collapsed.
Preoperative Planning
Little preoperative planning is necessary for this procedure. A recent magnetic resonance study of both hips should be obtained and examined to determine the size and location of the osteonecrotic lesion and to evaluate for lesions in the contralateral hip. Bilateral osteonecrosis of the hips occurs in up to 85% of patients, and most asymptomatic hips progress to symptomatic disease and/or femoral head collapse. Given the low morbidity of core decompression, surgeons may consider decompressing both hips if asymptomatic disease of the contralateral joint is identified on magnetic resonance imaging. In addition to the necessary instruments, surgeons should ensure that intraoperative fluoroscopy and a radiolucent table are available for the procedure.
Description of Techniques
Core decompression has traditionally been performed with trephines or cannulated drill bits that have diameters of between 5 and 10 mm. These instruments are driven through the femoral neck into the head under fluoroscopic guidance. Various materials, such as bone graft and osteogenic proteins, may be placed into the tracts, or the channels may be left open. Complications can occur if the drill hole is started too distally relative to the femoral metaphysis, which may result in a subtrochanteric fracture. Additionally, the drill hole may be extended too far, damaging the articular cartilage and leading to loose bodies within the joint capsule.
Technique: Modified Hungerford
• Position the patient supine on a fracture table.
• Use image intensification to track progress of the trephine in both anteroposterior and frog-leg lateral projections, ensuring that the cortices are not breached, and that the tip does not penetrate the articular cortex of the femoral head (Fig. 58-1).
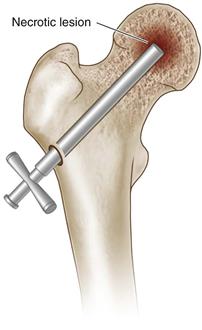
Figure 58-1 Introduction of an 8 to 10 mm trephine through a cortical window in the metaphysis of the proximal femur, advancing up the femoral neck into the necrotic lesion (indicated by the arrow).
• Once the lesion has been reached, the trephine is withdrawn along with the K wire and a core of trabecular bone (Fig. 58-2).
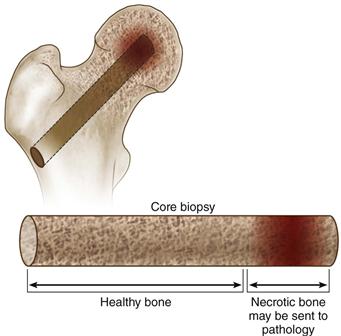
Figure 58-2 The trephine is withdrawn along with a core of both necrotic and viable cancellous bone. The necrotic material can be sent for histopathologic analysis.
A newer modified core decompression technique, which is really a percutaneous drilling procedure, has been described by Song and associates15 and Mont and colleagues16 and involves using a smaller-diameter drill bit or a Steinman pin (2 to 3 mm) inserted percutaneously to create the core tracts. Multiple drillings are created in an attempt to sufficiently decompress the diseased region. In addition, bone matrix or growth factors can be placed into the tracts. Potential advantages of this technique include percutaneous insertion rather than a surgical incision, shorter surgical time, and less tissue damage. In addition, this technique may be associated with a lower risk of fracture or collapse.
Variations and Unusual Situations
Core decompression is a relatively simple and safe procedure that can be used in virtually all patients with the appropriate indications. Use of intraoperative fluoroscopy to guide the path of the intraosseous channel allows accommodation of virtually any anatomic variations of the proximal femur, such as hip dysplasia, slipped capital femoral epiphysis, post-traumatic anatomic changes, or a pistol grip deformity of the proximal femur. Regardless of the femoral anatomy, surgeons should ensure that the femoral cortex is not penetrated, and that the decompression tract reaches the lesion in the subchondral space.
Steinberg and coworkers described a variation of the modified Hungerford technique.17 After the initial core tract is made, two additional passes are made into other areas of the necrotic lesion through the same cortical entry point, using 5 or 6 mm trephines. Then, the large-diameter trephine is reintroduced with the aid of a trocar and is used as a guide to loosely place morselized cancellous bone and/or bone growth–stimulating substances into the decompressed lesion. The rationale for this procedure as described by the authors was to stimulate revascularization and bone growth.
Postoperative Care
Recommended postoperative care has been similar regardless of the decompression technique used. Patients are typically discharged home on the same day of the procedure or on the following morning. They are mobilized before discharge, using a single crutch or cane in the contralateral hand. In cases of bilateral hip decompressions, two crutches or canes are used. Patients are advised to maintain partial weight bearing until a follow-up visit at approximately 6 weeks after the procedure. Patients are then allowed to progress to full weight bearing as tolerated but are counseled to avoid high-impact activities for 12 months and are encouraged to perform regular home-based abductor-strengthening exercises. One year after the core decompression procedure is performed, patients are evaluated clinically and radiographically for disease progression and/or femoral head collapse. If no evidence of disease progression is found, all restrictions are lifted, although patients are encouraged to return for regular annual follow-up examinations to monitor for radiographic signs of delayed disease progression.
Results
Over the past several decades, considerable variability has been noted in reported results of various decompression techniques for osteonecrosis of the femoral head. The literature includes a paucity of well-designed comparative studies and considerable heterogeneity in study populations in terms of lesion size, location, and radiographic stage. Reporting of results is further complicated by differences in the definition of successful and/or failed treatment, with different authors alternately measuring success as preservation of the native joint, no radiographic progression of disease, or no symptomatic progression.
Overall results of core decompression reported over the last decade as measured by the avoidance of further surgery range from 42% to 86% at mean follow-up times from 24 to 94 months, with most studies reporting survival rates in the range of 70% to 85% over this period (Table 58-2).15,18-31 Success rates as measured by an absence of radiographic progression are more variable and somewhat more modest, ranging from 30% to 86% over a similar range of follow-up times. Several studies have consistently reported that superior survival rates are seen in earlier radiographic stages of the disease (stages I and II) and with smaller, more medially located lesions.15,16,23,30,32-34
Table 58-2
Recent Studies Reporting Outcomes of Core Decompression and Percutaneous Drilling
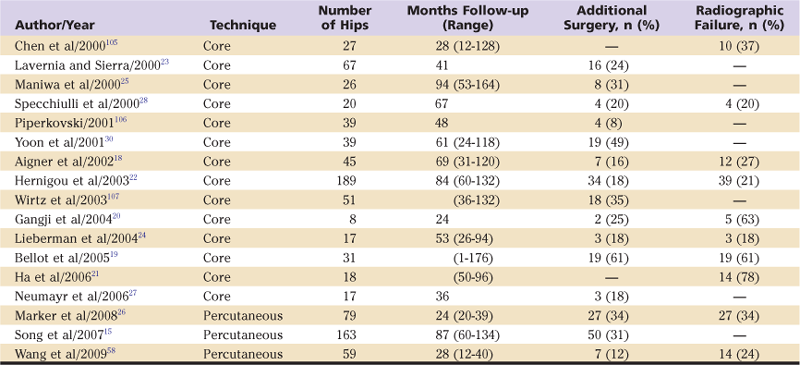
Some authors raise concerns about the degree, if any, to which core decompression affects the natural course of the disease,27,35-37 noting that many small early-stage lesions do not progress, even without surgical treatment. One well-controlled study did not confirm an effect with core decompression.27 However, several comparative studies and literature reviews have demonstrated consistently poorer survival rates for hips treated nonoperatively and overall failure rates approximately double those seen with core decompression.38–40
In summary, although a number of patients treated with core decompression eventually do progress to further surgical treatment, this procedure is minimally invasive (especially when performed using the percutaneous small-diameter technique), can be performed quickly with minimal technical requirements, has very low morbidity, provides immediate symptomatic relief in the large majority of patients, and may successfully delay further surgical treatment for many years in many patients who otherwise are offered much larger procedures, if not joint arthroplasty. For these reasons, the authors advocate the use of percutaneous core decompression for the treatment of symptomatic precollapse small to medium-sized lesions.
Complications
The principal risks of core decompression are related to the anesthesia of the surgery because the procedure itself has few complications. Authors experienced in core decompression typically report complication rates well below 1%. Patients treated with large-diameter trephine core decompression are at risk for proximal femur fracture should a fall occur in the early postoperative period, when the proximal femur is structurally weakened and the bone has not yet fully healed (Fig. 58-3). Improper placement of the trephine in the diaphysis of the femur can account for most fractures. However, the rate of these complications is low and can be further minimized by ensuring that an appropriate entry point is used in the metaphysis opposite to the proximal margin of the lesser trochanter, and that a period of postoperative protected weight bearing is strictly followed.
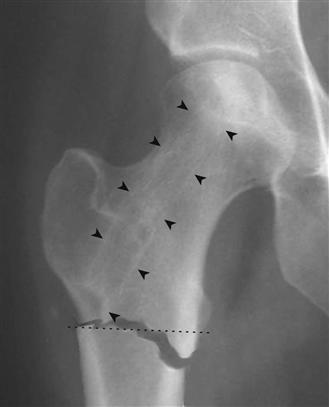
Figure 58-3 Subtrochanteric fracture involving the lateral cortical entry point of a recent core decompression tract (arrowheads). Note that the entry point is below the level of the proximal lesser trochanter (dashed line), which increases the risk of postoperative fracture. (Copyright Mount Sinai Hospital Inc., Baltimore, Md.)
Structural Bone Grafting
Structural bone grafting is a more invasive procedure than core decompression, so it is typically utilized for patients who have failed core decompression, or who would not be appropriate candidates for core decompression owing to large lesions or more advanced stages of disease. The general procedure for bone grafting involves soft tissue dissection to access the femur, removal of a window of cortical bone to access the intramedullary space, débridement of the necrotic bone, placement of the bone graft material, replacement of the bone window, and wound closure. Various types of bone grafting material can be used, including nonvascularized iliac crest, fibula, or tibia autograft or allograft; synthetic bone graft material such as demineralized bone matrix and corticocancellous bone chips; or vascularized fibular or muscle-pedicle autograft. In addition, growth factors such as bone marrow and bone morphogenetic proteins 2 and 7 have been added to the graft material in an attempt to stimulate new bone growth. Although a number of procedures have been described, they can be broadly divided into two categories: nonvascularized and vascularized bone grafting. Additionally, a few recent reports have described implantation of nonbiological porous tantalum metal rods, which may act like a structural bone graft. The rationale for bone grafting is multifold: (1) The technique provides a mechanism to decompress the femoral head, reducing intramedullary pressure; (2) it removes some or all of the necrotic bone, eliminates a source of inflammation, and provides a more suitable environment for the growth of viable bone; (3) it provides structural support with bone graft for the articular surface to prevent further collapse; and (4) it provides a mechanism for the placement of bone growth factors to stimulate healing. An additional rationale for the use of vascularized bone grafting is to introduce healthy viable bone into the lesion as a replacement for necrotic tissue.
Nonvascularized Bone Grafting
Indications and Contraindications
Indications for nonvascularized bone grafting include a Ficat stage II or III lesion with less than 2 mm of femoral head collapse. Failure of core decompression to adequately address the symptoms of osteonecrosis might also be an indication. It should not be used in patients who have articular cartilage defects that are greater than 1 cm in diameter, delaminated cartilage, acetabular changes, or very large areas of necrosis (>30% of the head) because they will not have high success rates with this procedure.
Preoperative Planning
Preoperative planning should include magnetic resonance imaging and/or plain film radiographs of the affected hip to accurately define the size and location of the necrotic area. This is important so that the necrotic lesion can be approached most directly with any type of nonvascularized bone grafting. The surgeon should additionally ensure that sufficient graft material is available if bone allograft is being used.
Description of Techniques
Three different techniques have been described for nonvascularized grafting of the femoral head: (1) Phemister, (2) trapdoor, and (3) lightbulb techniques. The Phemister technique, the first one reported, was used originally for the treatment of post-traumatic osteonecrosis.41 It consisted of creating two core decompression tracts with 8 to 10 millimeter trephines and placing a core of graft material into the tracts. Modifications of this technique were reported by a number of authors for nontraumatic osteonecrosis,42–45 including the use of a single core decompression tract and the use of various graft sources such as tibial, fibular, or iliac crest autografts and fibular allograft.
Modified Phemister Technique
• Press-fit the graft into the decompression tract, using fluoroscopy to confirm placement and ensuring that the rounded proximal end is in the subchondral space (Fig. 58-4).
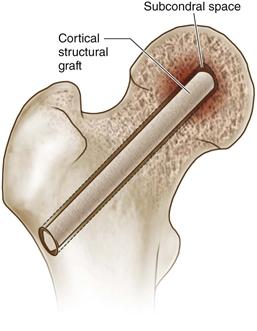
Figure 58-4 The Phemister technique for bone grafting involves placing a cortical structural graft into a core decompression tract, ensuring that the rounded end of the graft lies in the subchondral space of the decompressed lesion (indicated by the arrow).
To improve visualization and débridement of the necrotic lesion, Meyers and associates proposed introducing iliac cancellous autograft through a cortical window in the articular surface of the femoral head, directly above the necrotic lesion.46,47 This approach was later modified by Mont and colleagues, who described the use of iliac cancellous and cortical graft through an articular trapdoor.48
Trapdoor Procedure
• The window is cut by using an oscillating saw and angling the blade toward the center of the trapdoor while cutting to create a beveled edge. Once all four sides have been cut, the osteochondral trapdoor is removed and retained (Fig. 58-5).
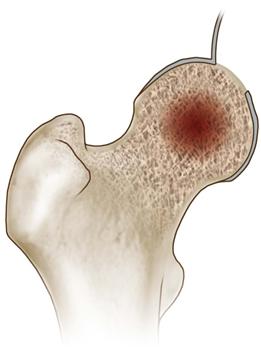
Figure 58-5 A trapdoor is created in the articular surface of the femoral head and the subsequent flap is lifted utilizing either scalpel dissection (pictured) or a small oscillating saw (instrument not pictured).
• Using a high-speed burr and curettes, all necrotic bone is removed until bleeding cancellous bone is visualized throughout the base of the lesion (Fig. 58-6A and B). A penlight can be periodically inserted into the cavity to improve visualization and ensure that all necrotic bone has been removed.
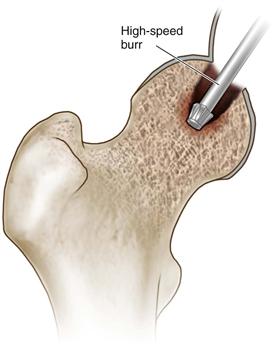
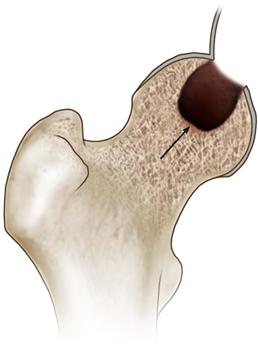
Figure 58-6 Using a high-speed burr, top thoroughly débride the necrotic lesion, bottom ensuring that bleeding cancellous bone is visualized at the base of the newly formed cavity (indicated by the arrow).
• Two or three cortical struts are impacted into the base of the intraosseous cavity (in the technique reported by Meyers, only cancellous bone was used). Fill the remaining void with cancellous bone, with added demineralized bone matrix if desired, and replace the osteochondral trapdoor; fix it in place using three absorbable pins (Fig. 58-7).
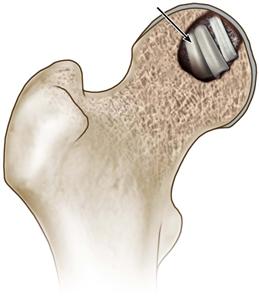
Figure 58-7 Impact cortical struts into the base of the cavity (indicated by the arrow), fill the remaining void with morselized bone graft, and replace the trapdoor, fixing it with absorbable pins.
• Reduce the hip joint and close the wound in layers over a drain.
Rosenwasser and associates reported a modification of the trapdoor technique known as the lightbulb procedure, which allows access to the necrotic lesion in the femoral head for débridement and bone grafting without the need for dislocation of the hip joint.49
Lightbulb Procedure
• Create a trapdoor in the anterior femoral neck, just distal to the articular surface of the femoral head, making beveled cuts using a small oscillating saw, as described for the trapdoor technique (Fig. 58-8).
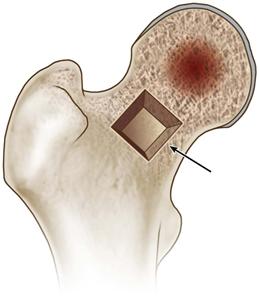
Figure 58-8 Using a small oscillating saw, create a trapdoor in the anterior femoral neck, just distal to the articular surface of the head (as indicated by the arrow). (Modified from Seyler TM, Marker DR, Ulrich SD, et al: Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res 466:1125–1132, 2008. Used with permission.)
• Using a high-speed burr and curettes, thoroughly débride the necrotic lesion all the way to the subchondral bone of the femoral head (Fig. 58-9A), using a penlight to enhance visualization and ensure that bleeding cancellous bone is present at the base of the cavity (Fig. 58-9B).
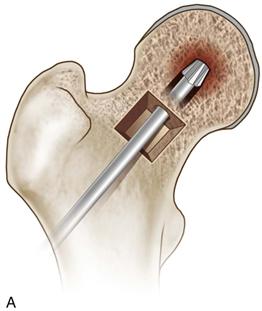
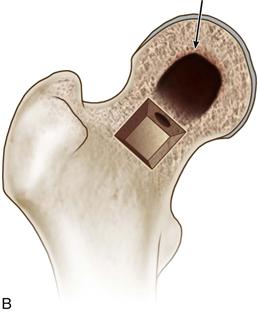
Figure 58-9 A, Use a high-speed burr and curettes to thoroughly débride the necrotic lesion, (B) ensuring that bleeding cancellous bone is visualized throughout the newly formed cavity (indicated by the arrow). (Modified from Seyler TM, Marker DR, Ulrich SD, et al: Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res 466:1125–1132, 2008. Used with permission.)
• Replace the cortical trapdoor, fixing it in place with three absorbable pins (Fig. 58-10).
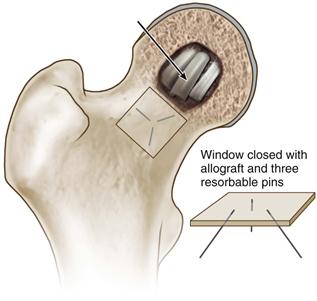
Figure 58-10 Pack the cavity with cortical and/or cancellous bone graft (indicated by the arrow), and replace the cortical window, fixing it in place with three absorbable pins. (Modified from Seyler TM, Marker DR, Ulrich SD, et al: Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res 466:1125–1132, 2008. Used with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







